There has been a growing understanding of the microbial threats that exist in laboratory & medical practice as of today. Simultaneously these threatening trends have been accompanied by a tremendous advance in the science and technology of in-clinic sterilization.
There has been a growing understanding of the microbial threats that exist in laboratory & medical practice as of today. Simultaneously these threatening trends have been accompanied by a tremendous advance in the science and technology of in-clinic sterilization.
All Medical Professionals must possess a good basic knowledge about the process of sterilization as otherwise their patients’ as well as their staff & their own health & lives are at risk.
So what exactly is sterilization?
Sterilization is the destruction or removal of all viable microorganisms, including many of the resistant bacterial spores. Since this process covers the broad range of pathogens that may be found in dental, ophthalmic & any surgical procedure, it is the only clinically acceptable means for dealing with reusable instruments between patients.
In order to understand “Why Class B Autoclave?”, let us first understand how a Steam Pressure Steriliser – commonly known as an Autoclave – sterilizes.
Autoclaves commonly sterilize by exposing its charge (items to be sterilized) to elevated temperatures of 121 to 134ºC under pressure of 15 to 30 psi, for a holding time of anywhere between three to 30 minutes. Please note that the three to thirty minutes time is the holding time at the set elevated temperature & not the total process or cycle time. Total cycle time would be far greater. The combination of the three lethal parameters of time, temperature and steam, deliver a powerful kill rate which even the hardiest of bacteria find hard to survive. This effective & yet clean method of lethality is unmatched by any of the other methods of sterilisation.
Saturated steam at this temperature is an excellent carrier of heat. The operative word is saturated steam. The steam condenses onto its charge and as it does so it not only expends its huge latent heat but also draws in additional steam to replace the condensed steam. Thus the heat transfer is very efficient and the penetration levels extremely high.
However, if there is residual air in the chamber and load, it will interfere with steam-instrument contact, and may compromise sterilization. This residual air can prevent penetration of steam to the depths of the load, leaving your sterilisation incomplete. Why & how would this happen? When you load the Autoclave with instruments and close the lid, there is already a lot of stale air trapped inside. For sterilisation to take place, this air needs to be effectively purged and replaced with saturated steam.
To resolve this problem of purging entrapped air, normal Autoclaves will have a manual, mechanical or electrically operated valve open till about 100ºC. Once it is closed the pressure & temperature begins to rise. It is presumed that by this time, the entire entrapped inner air has been purged. However, tests have shown that this is not an effective method for vials, wrapped items, implants, garments and certain types of hollow ware. You can never be assured of efficient penetration of steam right inside, if you are using a regular Autoclave. Even a small volume of entrapped air can compromise your sterility assurance level simply because ordinary entrapped air is a very bad conductor of heat & moisture. These pockets of air cannot conduct heat to the load with the same vigor as steam and therefore cold spots remain within the load.
The type N-cycle is suitable for sterilizing only unwrapped and solid instruments. N-cycle sterilizers are the most popular bench top autoclaves (top or front loading), and are classified as passive systems (also known as gravity or non-vacuum). Typically, as steam is generated or admitted into the sterilization chamber, it forces unsaturated and saturated air out through a vent. The major concern with the N-cycle sterilizers is the non-removal of trapped air (especially air pockets in lumens and difficult-to-access areas of the load) during displacement.
Errors in packaging or overloading the sterilizer chamber can result in cool air pockets where items are not sterilized.
The type B sterilisation cycle is used to process loads that can retain air. It can sterilize solid, hollow, and porous instruments, be they wrapped or unwrapped. Pressurized steam is an effective sterilizing agent providedthat the internal air has first been removed from the chamber as well as the air retentive load. Residual air prevents direct contact between steam & the load to be sterilised. Hence this B-cycle involves the use of active (forced) air removal, usually by using an inbuilt vacuum pump. These pre vacuum or Class-B sterilizers create a vacuum, thus removing air from the load prior to the chamber being pressurized with steam. This can be done from 1 to 3 times – depending on the cycle selected. This technique allows faster and more effective steam penetration throughout the entire instrument load than the gravity displacement technique typically employed in sterilizers with N-cycles.
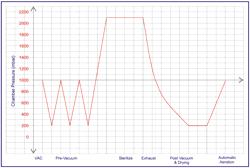
A typical cycle of a Class B Sterilizer:
A Class-B Autoclave is essential if you are:
- Pouching your instruments
- Double wrapping your instruments
- Sterilising lumens, implants or instruments that have difficult to access areas
- Sterilising porous loads like garments, gowns, dressings sterilizing
- Hollow ware of Type A Sterilising Vials
Contact Us:
E-mail: hs@medicainstrument.com
Ph: 022-66189999
http://www.medicainstrument.com























