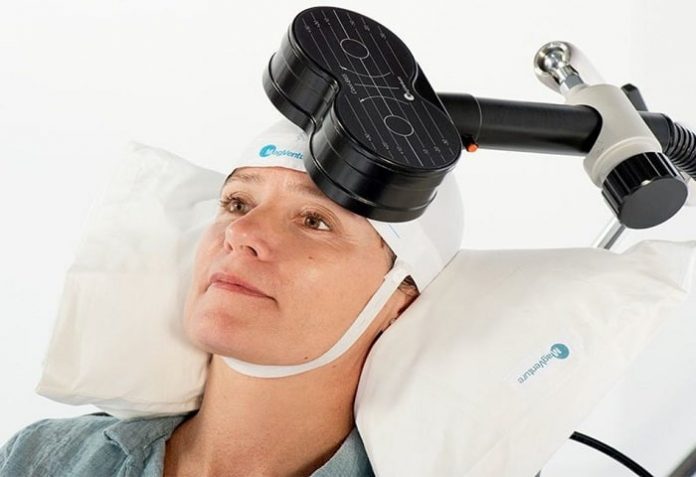
MagVenture A/S, a global medical device company, has established a subsidiary in the UK. MagVenture specializes in magnetic stimulation, a technology used within the fields of neuro-diagnostics and research as well as for the treatment of depression.
We are proud to now be represented with a UK subsidiary. This will allow us to fully meet our customers’ needs and offer direct representation within sales, marketing and support,” says Samuel Jacobs, National Sales Manager of MagVenture Ltd.
Our UK subsidiary, based in Reading, will not only help us be closer to the UK market but also ensure that we can provide our internationally recognized high-level support for our growing number of customers,” says Samuel Jacobs, who especially sees a growing interest within the non-invasive magnetic stimulation markets in the UK.
Transcranial Magnetic Stimulation (TMS) allows researchers to stimulate the human brain, noninvasively.TMS does not only provide vital information and evidence on how the human brain works, but also leads to new treatments and therapies within neurology and psychiatry, such as for the treatment for depression. TMS, when used for the treatment of depression, delivers magnetic pulses to stimulate nerve cells in the part of the brain controlling mood, an area often underactive in depressed patients.
Major Depressive Disorder (MDD) is a leading cause of disability worldwide and although several treatment options are currently available, not all patients will benefit from them; In fact, approximately 60% do not respond adequately to conventional treatments such as antidepressants and are therefore in dire need of a treatment alternative. TMS is known to produce an antidepressant effect in over 60% of these hard-to-treat patients.
MagVita TMS Therapy for the treatment of MDD is CE approved in the UK and Europe. Full treatment guidance was provided in 2015 by the National Institute for Clinical Excellence (NICE) for the treatment of both MDD and Migraine. TMS for the treatment of MDD has an FDA clearance from 2008 and is commercially available in the US.
Further information:
Samuel Jacobs, National Sales Manager, MagVenture Ltd., UK.
02030 869 066 / infouk@magventure.com, www.magventure.co.uk
MagVenture A/S is a medical device company with over 25 years of experience within the neuromodulation sector. From its headquarters in Denmark, MagPro magnetic stimulators are promoted worldwide through direct sales subsidiaries in Germany, the USA and now in the UK, and through a global network of dedicated distributors in Europe, Asia, Middle East and the Americas.