America’s AI Action Plan: Transforming Healthcare, Medical, and Pharmaceutical Industries
The Trump Administration’s comprehensive AI Action Plan represents a pivotal moment for the healthcare, medical, pharmaceutical, and biopharmaceutical sectors. Released in July 2025, this strategic framework outlines ambitious policies designed to accelerate AI adoption across critical healthcare domains while maintaining safety standards and promoting American innovation leadership.
Addressing Healthcare’s Slow AI Adoption Challenge
The Action Plan explicitly acknowledges that healthcare is one of the most critical sectors in America and has been “especially slow to adopt [AI] due to a variety of factors, including a complex regulatory landscape, a lack of clear governance and risk mitigation standards, and distrust or lack of understanding of the technology.” The foundation for focused interventions aimed at removing these structural obstacles is laid by this awareness.
The plan’s response centres on establishing a “try-first” culture across American healthcare through coordinated federal efforts. This approach represents a significant departure from traditional regulatory caution, emphasising innovation acceleration while maintaining safety protocols.
Revolutionary Regulatory Sandboxes for Healthcare Innovation
A cornerstone of the Action Plan involves creating regulatory sandboxes or AI Centers of Excellence where healthcare researchers, startups, and established enterprises can rapidly deploy and test AI tools. These initiatives will be enabled by key regulatory agencies, most notably the Food and Drug Administration (FDA), working in partnership with the Department of Commerce through its AI evaluation initiatives at NIST.
The FDA’s role extends beyond traditional oversight to active participation in innovation facilitation. The agency has already begun implementing AI-assisted review processes, with Commissioner Dr. Martin Makary describing their pilot program as a “game-changer technology” that enables scientific review tasks to be completed “in minutes that used to take three days”. This transformation in regulatory efficiency could dramatically accelerate the approval timelines for AI-enabled medical devices and pharmaceutical products.
Comprehensive Standards Development Through NIST
The National Institute of Standards and Technology (NIST) emerges as a central coordinating body for healthcare AI standards. The plan directs NIST to launch domain-specific efforts in healthcare, convening public, private, and academic stakeholders to accelerate the development and adoption of national standards for AI systems. These efforts will specifically measure how AI increases productivity in critical areas including medical imaging, diagnostics, clinical workflow, and population health analytics.
Notably, the plan requires NIST to revise its AI Risk Management Framework to eliminate references to “misinformation, Diversity, Equity, and Inclusion, and climate change,” refocusing on objective truth and technical performance metrics. This revision signals a shift toward performance-based evaluation criteria for healthcare AI systems.
Advanced Scientific Dataset Development for Medical AI
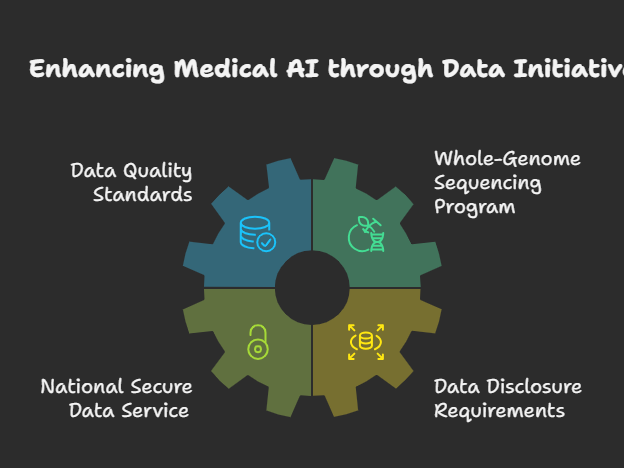
The Action Plan positions high-quality data as a “national strategic asset” and outlines extensive measures to build world-class scientific datasets for AI training. For healthcare applications, this includes several transformative initiatives:
The plan establishes minimum data quality standards for biological, materials science, chemical, and physical data modalities used in AI model training through the National Science and Technology Council. Additionally, it proposes creating a whole-genome sequencing program for life on Federal lands, which would generate valuable resources for training future biological foundation models.
A National Secure Data Service (NSDS) portal will expand access to non-sensitive, health-related datasets for AI model development, while federally funded researchers will be required to disclose high-quality datasets to support medical AI research. These measures address one of the most significant bottlenecks in healthcare AI development: access to comprehensive, high-quality training data.
Biosecurity and Pharmaceutical Safety Measures
Recognising the dual-use potential of AI in biological research, the Action Plan implements comprehensive biosecurity measures specifically relevant to pharmaceutical and biopharmaceutical industries. The plan requires all institutions receiving federal funding to use nucleic acid synthesis tools and providers with “robust nucleic acid sequence screening and customer verification procedures”.
This multi-tiered biosecurity approach includes enforcement mechanisms rather than voluntary attestation, ensuring that pharmaceutical research organisations maintain rigorous safety standards when using AI-powered biological design tools. The Office of Science and Technology Policy will convene industry and government stakeholders to develop systems for nucleic acid providers to share data on suspicious requests in real-time.
Workforce Development and Healthcare AI Skills
The Action Plan addresses the critical need for AI-literate healthcare professionals through comprehensive workforce development initiatives. A new federal hub will examine AI’s impact on healthcare jobs, including hiring trends, wages, and occupational demand. The Department of Labor will establish an AI Workforce Research Hub to evaluate AI’s impact on the healthcare labor market and generate actionable insights for workforce policy.
The plan also clarifies that AI literacy and skill development programs may qualify as eligible educational assistance under tax codes, enabling healthcare employers to offer tax-free reimbursement for AI-related training. This provision could significantly accelerate the adoption of AI skills among healthcare professionals.
Infrastructure and Cybersecurity Enhancement
Healthcare organisations will benefit from the plan’s broader AI infrastructure investments, including expanded data centers and improved grid access that may enhance AI processing capacity for health systems and academic medical centres. The Department of Homeland Security will launch an AI Information Sharing and Analysis Centre to help healthcare and other critical infrastructure sectors defend against AI-driven cyber threats.
These cybersecurity measures are particularly crucial given healthcare’s vulnerability to cyberattacks and the sensitive nature of medical data. The plan ensures that AI adoption in healthcare occurs within a robust security framework.
Federal Procurement and Healthcare AI Integration
The Action Plan mandates that all federal agencies ensure employees whose work could benefit from access to frontier language models have access to and appropriate training for such tools. For healthcare agencies like the Veterans Administration and the Centers for Disease Control and Prevention, this could accelerate the integration of AI tools into public health and clinical care delivery.
The plan also establishes an AI procurement toolbox managed by the General Services Administration, facilitating uniformity across the federal enterprise while allowing agencies flexibility to customise models for healthcare-specific applications.
Implications for Drug Discovery and Development
While not explicitly detailed in healthcare-specific sections, the Action Plan’s broader scientific research initiatives have profound implications for pharmaceutical and biopharmaceutical industries. The emphasis on AI-enabled science includes investments in automated cloud-enabled labs for biology, chemistry, and materials science. These facilities, built through collaborations between the private sector, federal agencies, and research institutions, could dramatically accelerate drug discovery timelines.
The plan’s support for Focused-Research Organizations using AI and emerging technologies for fundamental scientific advancements could revolutionize pharmaceutical research methodologies. Combined with requirements for researchers to disclose datasets used by AI models, this creates an ecosystem conducive to collaborative pharmaceutical innovation.
Global Competitiveness and Export Leadership
The Action Plan positions American healthcare AI as a global standard, with specific measures to export American AI technology to allies and partners while countering Chinese influence in international governance bodies. For pharmaceutical and medical device companies, this creates opportunities to establish American AI solutions as the international gold standard.
The plan’s emphasis on open-source and open-weight AI models provides particular advantages for healthcare startups and academic medical centres, allowing them to access cutting-edge AI capabilities without dependence on closed model providers. This democratisation of AI access could accelerate innovation across the entire healthcare ecosystem.
Conclusion
America’s AI Action Plan represents a paradigm shift for healthcare, medical, pharmaceutical, and biopharmaceutical industries. By combining regulatory innovation, infrastructure investment, workforce development, and security measures, the plan creates a framework for unprecedented AI adoption across healthcare sectors. While maintaining necessary safety standards through biosecurity measures and rigorous evaluation frameworks, the plan’s emphasis on speed, innovation, and global competitiveness positions American healthcare industries to lead the worldwide AI transformation. The success of these initiatives could fundamentally reshape how healthcare is delivered, how pharmaceuticals are developed, and how medical research is conducted, ushering in what the plan describes as a “new golden age of human flourishing” in healthcare.



















