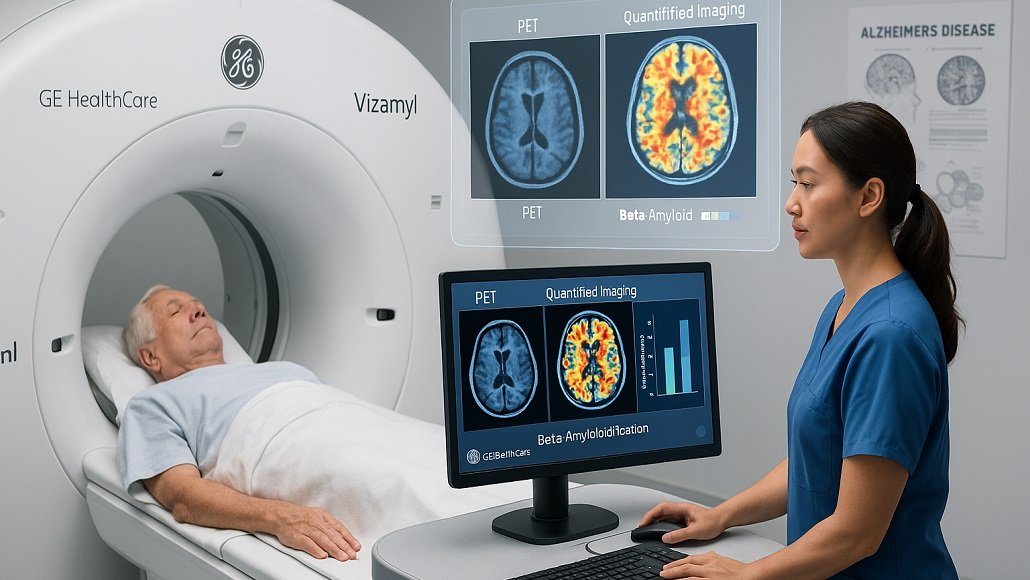
The US Food and Drug Administration (FDA) has gone on to expand the indications when it comes to an imaging agent that is developed by GE HealthCare, which is going to most likely put it at the forefront when it comes to the Alzheimer’s diagnostic space.
Apparently, the label expansion when it comes to GE HealthCare’s positron emission tomography (PET) imaging agent, Vizamyl, now happens to include qualification of amyloid within the brain, thereby meaning that patients taking an anti-amyloid therapy can be tracked for the effectiveness of the drug.
At present, there are two anti-amyloid therapies that are approved in the US, namely Kisunla from Eli Lilly and Leqembi by Eisai/Biogen. In spite of the reimbursement challenges when it comes to both the drugs, revenue for them is anticipated to surge in the decade to come. But patients who are looking to take them have to meet the strict criteria, and the therapy’s ongoing use comes with some caveats, all of which have to be diagnostically tracked with PET imaging.
It is well to be noted that the radiology professor, Philip Kuo, who happens to be from the City of Hope National Medical Center, says that the usage of qualification in terms of amyloid PET imaging has, as a matter of fact, steadily moved from research to clinical practice, in which it can aid in a much more confident as well as accurate diagnosis.
He adds that now quantification can also play a very crucial role in terms of initiating as well as tracking amyloid-targeted therapy when it comes to Alzheimer’s disease and, by way of it, determining when it can get discontinued.
Apparently, Vizamyl was first approved in 2013 so as to estimate beta amyloid neurotic plaque density within adult patients having cognitive impairment. Now the visual estimation has gone on to turn into quantification by opening more diagnostic avenues as far as the Alzheimer’s imaging agent is concerned.
In yet another boost when it comes to patients, the label expansion also helps in adding up an explicit indication for the selection of patients who are eligible for the therapy. Effectively, the expansion now, in a way, enables the diagnosis of Alzheimer’s. This has been based on the revised criteria coming from the Alzheimer’s Association, which indicates that an abnormal amyloid PET scan is sufficient enough so as to establish a diagnosis.
It is well to be noted that this updated label also eradicates a previous restriction that predicted cognitive decline or even progression to dementia, thereby reflecting novel evidence that goes on to link amyloid-positive scans to higher risk in terms of advancing from early mild cognitive impairment to dementia in Alzheimer’s.
Although there are certain other available amyloid diagnostics like Lilly’s Amyvid, these do not have a similar current range when it comes to label indications as what Vizamyl has. Interestingly, GE HealthCare also has some of the most advanced PET devices on the market. As per an analysis done by GlobalData, the company happens to have the largest global market share when it comes to nuclear imaging equipment, under which PET scanners happen to be a segment.
Jit Saini, who happens to be the pharmaceutical diagnostic division’s chief medical officer at GE HealthCare, says that the inclusion when it comes to quantification as well as the removal of the therapy tracking limitation from the Vizamyl label is indeed very good news for healthcare providers as well as their patients, thereby further helping with timely as well as appropriate decisions pertaining to care.
The fact is that research within Alzheimer’s diagnosis is indeed gaining a lot of traction amid the revenue ceiling when it comes to anti-amyloid therapies. In May 2025, the FDA went on to approve the Lumipulse G Test, which happens to be the first blood test in order to help with the diagnosis of the disease. The agency said that the new tool can also help in reducing the number of PET scans, which are very expensive as well as radiation-exposing.
The tests are developed by Fujirebio Diagnostics, and they measure the ratio between tau and amyloid proteins within the blood and also tie the findings to the likelihood of discovering plaque buildups within the brain.