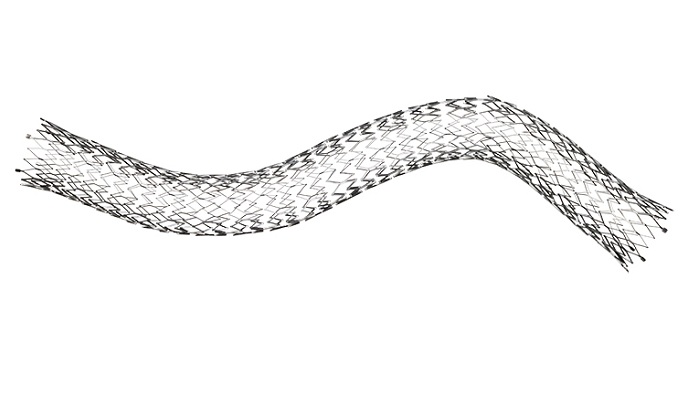
BD , a leading global medical technology company, announced the U.S. FDA has granted premarket approval for the Venovo™ venous stent, the first stent indicated to treat iliofemoral venous occlusive disease, which is obstructed or narrowed blood flow specific to the iliac and femoral veins located near the groin.
The Venovo™ venous stent is a flexible nitinol stent specifically designed to reopen blocked iliac and femoral veins in order to maintain adequate blood flow. The Venovo™ venous stent is designed with a balance of radial strength, compression resistance and flexibility needed for the treatment of symptomatic post-thrombotic and non-thrombotic iliofemoral lesions. Additionally, the broad stent sizing allows clinicians to treat large diameter veins and long lesion lengths.
“The unique attributes of the Venovo stent make it particularly well-suited to treat iliofemoral occlusive disease,” said Dr. Michael Dake, University of Arizona and the principal investigator for the Venovo IDE trial. “Most importantly, it is purpose-built for application in veins, and engineered to address the special challenges of venous lesions that are very different than those posed by arterial narrowing.”
Iliofemoral venous occlusive disease occurs when there is impaired blood flow in the iliofemoral vein caused by acute or chronic deep-vein thrombosis, post-thrombotic syndrome, iliofemoral vein compression including May-Thurner Syndrome or a combination of these diseases.1 Symptoms include swelling of the legs, pain when standing, skin discoloration and ulcers.2
One-year results from the prospective, multicenter single-arm VERNACULAR trial involving 170 subjects demonstrated the safety and effectiveness of the Venovo™ venous stent for the treatment of symptomatic iliofemoral venous outflow obstruction. The clinical findings showed a weighted primary patency rate of 88.3 percent, with a 96.9 percent patency rate in non-thrombotic lesions and an 81.3 percent patency rate in post-thrombotic lesions at 12 months, exceeding the performance goal of 74 percent. In addition, patients treated with the Venovo™ venous stent reported a statistically significant reduction in pain symptoms and improvement in quality of life (assessed by CIVIQ-20) at 12 months from baseline. The Venovo™ venous stent was also deployed successfully to the target lesion and showed adequate coverage in all cases, and there were no fractures seen at 12 months.
“The FDA premarket approval of the Venovo venous stent represents a significant advance for interventionalists treating iliofemoral venous occlusive disease, an underrecognized condition,” said Steve Williamson, worldwide president of Peripheral Intervention at BD. “We designed the Venovo venous stent in collaboration with clinicians to enable them to treat both post-thrombotic and non-thrombotic lesions. Clinicians will now have access to the broadest range of stent sizes in the U.S. for these difficult-to-treat lesions.”
The Venovo™ venous stent is commercially available in the U.S., Europe, Argentina, Australia, Brazil, Egypt, India, Israel, Mexico, Russia, Saudi Arabia, Singapore and Taiwan.
About BD
BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. The company supports the heroes on the frontlines of healthcare by developing innovative technology, services and solutions that help advance both clinical therapy for patients and clinical process for healthcare providers. BD and its 65,000 employees have a passion and commitment to help enhance the safety and efficiency of clinicians’ care delivery process, enable laboratory scientists to accurately detect disease and advance researchers’ capabilities to develop the next generation of diagnostics and therapeutics. BD has a presence in virtually every country and partners with organizations around the world to address some of the most challenging global health issues. By working in close collaboration with customers, BD can help enhance outcomes, lower costs, increase efficiencies, improve safety and expand access to healthcare. In 2017, BD welcomed C. R. Bard and its products into the BD family. For more information on BD, please visit bd.com.