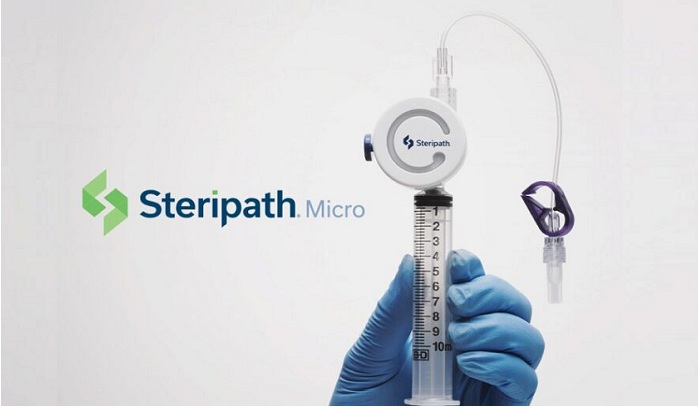
Magnolia Medical Technologies, Inc. – the trusted solution for reducing blood culture contamination announced the launch of its new Steripath® Micro Initial Specimen Diversion Device®. Micro is the first FDA 510(k)-cleared device indicated to reduce blood culture contamination,1 designed to be used with the most vulnerable patient populations.
Working closely with leading hospitals throughout the country, Magnolia developed the new lightweight, lower diversion volume, easy-to-use Steripath Micro with syringe-driven diversion and collection. The new device leverages the trusted ISDD®technology while diverting less than 1.0 mL of blood, engineered for patients with low-blood volume and difficult venous access.
“We are pleased to introduce the Steripath Micro platform as an integral part of our expanding ISDD portfolio designed for use with our most precious patient populations,” said Greg Bullington, CEO of Magnolia Medical. “This new product introduction has broadened the Steripath portfolio, enabling us to address the wide variety of use cases associated with blood culture collection. We are committed to establishing the new standard of care for sepsis testing accuracy for all patient populations with a focus on preventing patient harm by reducing false-positive diagnostic test results.”
Specifically, the Steripath Micro architecture uses syringe-driven negative pressure to divert and sequester the initial 0.6 to 0.9 mL of blood. Once diversion is complete the user simply presses a button to isolate the diverted blood and automatically a second independent blood flow pathway opens to collect the blood specimen for culture into the syringe.
The patented Steripath® ISDD® product portfolio, including the Steripath Gen2 direct-to-media and syringe configurations in addition to Steripath Micro, are the only FDA 510(k)-cleared devices indicated to reduce blood culture contamination.1
Steripath is a clinically proven solution to address a significant hidden problem in healthcare: the misdiagnosis of sepsis.2,3Improving the accuracy of diagnostic test results for sepsis can reduce unnecessary antibiotic treatment. This helps to address the growing threat of antibiotic-resistance, decreases hospital length of stay and associated healthcare-acquired infections while significantly reducing avoidable hospital costs.5,6
Magnolia Medical Technologies develops, manufactures and markets innovative blood and bodily fluid collection devices to facilitate significant improvements in the accuracy, consistency and predictability of critical laboratory tests. Magnolia Medical invented and patented the initial specimen diversion technique (ISDT™) and device (ISDD®) for blood culture collection and contamination prevention. The company has amassed an intellectual property portfolio including more than 70 issued method, apparatus and design patents with more than 50 additional patent applications pending.