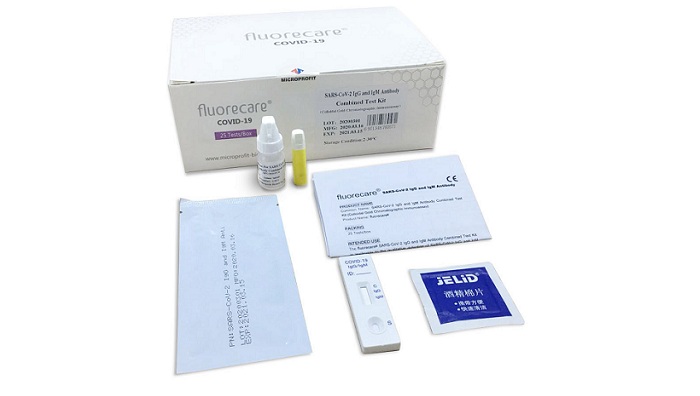
NovaBay Pharmaceuticals, Inc. announces an agreement with Shenzhen Microprofit Biotech Co., Ltd. to become the exclusive U.S. distributor of a rapid, finger prick test to determine the presence of COVID-19 or a potential indication of antibody immunity to COVID-19. The fluorecare® SARS-CoV-2 IgG & IgM Antibody Combined (colloidal gold chromatographic immunoassay) Test Kit is a point-of-care test to be administered by healthcare professionals. The test uses a drop of blood for the detection of COVID-19 antibodies with results available in approximately 10 minutes.
The fluorecare test kit has been validated through widely used RT-PCR testing to detect immunoglobulin M (IgM), which is the first antibody produced in response to initial exposure to the COVID-19 antigen, and immunoglobulin G (IgG), which provides a potential indication of antibody-based immunity to COVID-19. The fluorecare test kit has been ISO 13485 and CE Mark certified.
“Public health experts and leaders across our country are citing a critical need for mass testing and tracing procedures for those who are infected or have been infected with COVID-19 before reopening the nation’s economy,” said Justin Hall, NovaBay CEO. “Nasopharyngeal (back of the nose and throat) swabs for molecular detection are expensive and require laboratory testing that can lead to delays in obtaining results. Through a simple finger prick, IgG/IgM testing could provide for cost-effective detection of COVID-19 antibodies with results available in minutes as an important step in tracking the infection.
“We are delighted once again to work with our global health supplier network to secure a product that can help our communities during the COVID-19 pandemic and, subject to FDA clearance, we plan to offer the fluorecare test kit at very competitive pricing,” he added.
NovaBay will submit the fluorecare test kit to the U.S. FDA under Emergency Authorization Use (EAU), which will be effective until the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 is terminated. The Company will also submit the fluorecare test kit for permanent FDA 510(k) clearance so that the test kit can continue to be used once the state of emergency has been declared over by the Federal Government. Because these test kits are one of the first few test kits of its kind to be reviewed by the FDA, NovaBay cannot assure a timeline for FDA review and/or clearance for commercial marketing of the fluorecare test kit in the U.S. under EAU or 510(k), or if clearance will be granted at all.
About NovaBay Pharmaceuticals, Inc.:
NovaBay Pharmaceuticals, Inc. is a biopharmaceutical company focusing on commercializing and developing its non-antibiotic anti-infective products to address the unmet therapeutic needs of the global, topical anti-infective market with its two distinct product categories: the NEUTROX® family of products and the AGANOCIDE® compounds. The Neutrox family of products includes AVENOVA® for the eye care market, CELLERX® for the aesthetic dermatology market, and NEUTROPHASE® for wound care market.