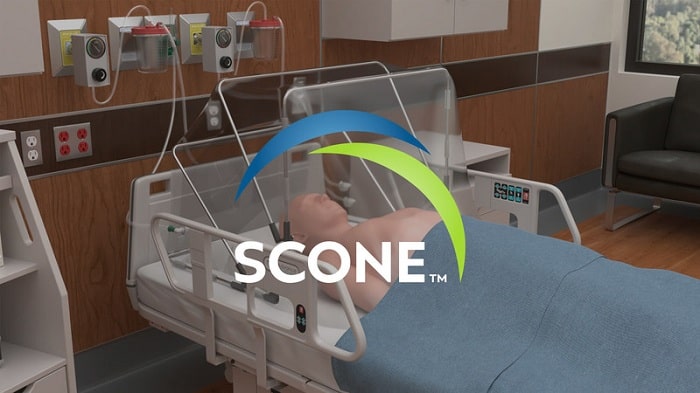
As the world faces a new era of emerging and re-emerging infectious diseases, new technologies are paving the way for safer, more effective treatment options. The Self-Contained Negative Pressure Environment is a new technology developed by SCONE Medical Solutions Inc. (SMS) in collaboration with Mayo Clinic for infectious disease containment in hospitals. The FDA recently granted Emergency Use Authorization to the SCONE™ device on December 18, 2020.
The highest risk of infectious transmission is from actively infected patients to health care workers (HCWs) during Aerosol Generating Procedures (AGPs), particularly in the acute care/triage setting. The SCONE™ is a small capacity, disposable device that uses negative pressure to vacuum out aerosols emitted around a patient’s head and neck, adding an extra layer of “active” barrier protection for healthcare workers while treating potentially infectious patients. The SCONE™ can be quickly deployed for use and quickly disposed of after treatment.
Mike Adams, CEO of SCONE Medical Solutions Inc. (SMS) says, “We are pleased to collaborate with Mayo Clinic experts as we bring the SCONE™ device to market. The demand for barrier protection in hospitals has shown itself in a substantial way during this pandemic. The SCONE™ works not only as a protective barrier, but through the use of negative-pressure, actively reduces the spread of pathogenic aerosolized particulates that cause diseases like COVID-19.”
During the spring of 2020, the SMS team ramped up efforts to begin designing, developing, testing the new SCONE™ device, and then in the fall prepared to launch a full-scale manufacturing process based in North Carolina. Now with the FDA EUA greenlight, SMS will begin distributing SCONE™ units across the country at the end of the year.
Dr. Brandon Lawrence, SCONE Chief Medical Officer and ER Physician in Phoenix, AZ, has been treating COVID-19 patients from the beginning. He states, “The widespread demand from HCWs for barrier protection devices, even the old ones without negative pressure, was overwhelming during the start of the pandemic. Adding negative-pressure technology allows patients to be more safely placed on CPAP/BIPAP, receive aerosolized nebulizer treatments, undergo emergent procedures if their COVID-19 status is unknown, and possibly allow end of life care visits with family.”
According to Michael Wallace, M.D. at Mayo Clinic, who collaborated with the SMS team: “There is an urgent need for small capacity self-contained negative pressure environments that utilize existing hospital suction lines and HEPA filtration. The development of the SCONE™ device will provide new opportunities for hospitals to control aerosol spread to other patients and healthcare workers.”
During the midst of this recent pandemic surge and beyond, SCONE™ aims to help ease the burden on hospitals by providing backup small-capacity negative pressure units that allow for safer treatment, triage, and patient transport. Implementing new, more sustainable, protocols using the SCONE™ device may also allow for planned family visitation and end-of-life care closure for those in desperate need of it most right now.