Government Unveils Life Sciences Roadmap for Growth and Innovation
London, July 2025 – The UK Government has officially launched its new UK Life Sciences Sector Plan, marking a watershed moment for an industry hailed as one of the nation’s greatest assets. With cross-party support and input from over 250 organisations and 400 individuals across life sciences businesses, the plan sets out a sweeping vision to reinforce the UK as a leader in global health innovation. While ensuring that scientific breakthroughs deliver tangible benefits to patients, the NHS, and the wider economy.
In a joint ministerial foreword, Secretary of State for Science, Innovation, and Technology Peter Kyle, Secretary of State for Business and Trade Jonathan Reynolds, and Secretary of State for Health and Social Care Wes Streeting expressed their determination to remove longstanding barriers such as slow commercialisation and regulatory bottlenecks that have historically hampered the sector’s enormous potential. “From hospital to community, from analogue to digital, and from sickness to prevention: these are the three major shifts this government is determined to deliver,” the ministers declared.
The Sector Plan: A Blueprint for the Next Decade
The Opportunity
- World-Leading Legacy: The UK’s life sciences industry boasts numerous world firsts—from pioneering vaccines and monoclonal antibodies to launching the first CRISPR therapy and leading personalised cancer vaccine research.
- Economic Engine: With over 300,000 people employed and £108.1 billion in turnover recorded for 2021/22, the sector’s growth is vital for national economic health. High-value jobs, a 13% annual increase in turnover, and diverse regional impact underpin its significance.
The Challenge
The new sector strategy addresses enduring obstacles:
- World-class at discovery, but weak on commercialisation and product adoption.
- Median setup times for clinical trials lag behind competitor nations.
- UK life sciences companies face difficulties scaling, partly due to limited access to venture capital and cautious domestic investors.
- Competition for international investment has intensified, especially post-pandemic, necessitating a shift from “incremental adjustment” to “comprehensive reform.”
Three Pillars of the Plan
The plan revolves around three interconnected pillars:
1. Enabling World Class R&D
- Record government R&D investment—over £2 billion allocated in this spending review.
- Focused funding for discovery science, translational models (including alternatives to animal models), and AI-driven biomedical research.
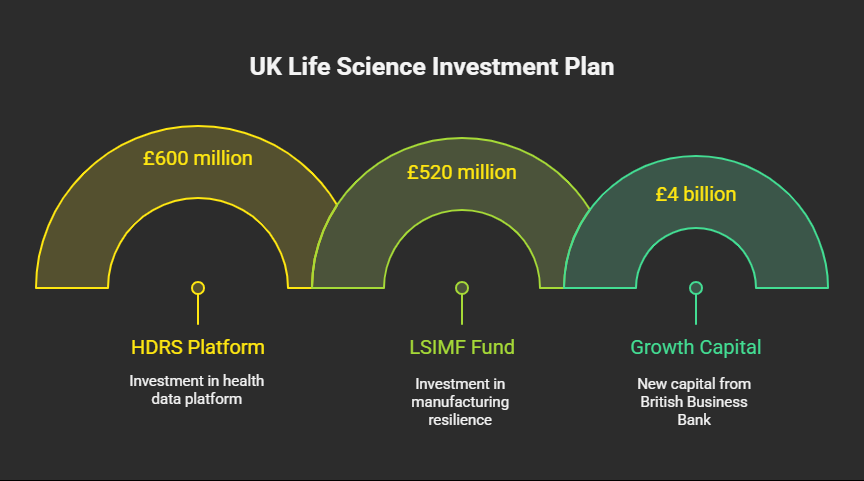
- Establishment of the Health Data Research Service (HDRS): a new £600 million platform for secure, AI-ready health data, making the UK a magnet for global clinical trials.
2. Making the UK a Hub for Startups, Scale-ups, and Investment
- Launch of the £520 million Life Sciences Innovative Manufacturing Fund (LSIMF) to attract manufacturing investment and build domestic supply chain resilience.
- Enhanced access to finance, including £4 billion in new growth capital from the British Business Bank to stimulate investment and crowd in private sector funding.
- Ambitious skills and talent strategies, diversity initiatives, and support for attracting top global researchers.
- Partnerships like the BioNTech agreement and dedicated services to help 10–20 high-potential UK companies scale domestically.
3. Driving Health Innovation and NHS Reform
- Commitment to streamline regulation, including reforms at the Medicines and Healthcare products Regulatory Agency (MHRA) and faster integration of innovations with the National Institute for Health and Care Excellence (NICE).
- Targets for reducing clinical trial setup times to under 150 days by March 2026.
- Innovation-friendly frameworks, a new “Innovator Passport” for medtech, and national procurement reforms to speed up patient access to new treatments and technologies.
- Delivering the NHS’s Net Zero Roadmap in partnership with industry.
Headline Actions and Targets
The government will prioritise six core actions for rapid impact:
- Building the HDRS health data platform
- Reducing clinical trial setup delays
- Backing manufacturing via LSIMF
- Streamlining regulation and market access
- Introducing low-friction procurement for medtech
- Creating new industry partnerships and support structures for growth firms
Progress will be tracked transparently with annual implementation updates and monitored by a refreshed Life Sciences Council comprising government, industry, and public stakeholders.
Key Targets by 2030–2035:
- Lead Europe in commercial R&D investment, scale-up capital, and foreign direct investment (FDI)
- Rank as the third most important global life sciences economy (behind only the US and China)
- Become one of the top three fastest regions in Europe for patient access to medicines and medical technology
Looking Forward
By 2035, the government envisions the UK as a powerhouse in life sciences, with:
- A robust ecosystem built from world-class R&D, investment, and manufacturing infrastructure.
- Nimble regulatory and market access frameworks, adopter-oriented NHS, and flourishing clusters from Cambridge to Glasgow.
- Increased capital markets capacity enabling more UK-based companies to grow into global firms.
- Enhanced and equitable health outcomes for patients nationwide, underpinned by early adoption of cutting-edge technologies, genomics, and personalised medicine.
The plan’s accountability mechanisms, annual reviews, and clear timelines are designed to sidestep the pitfalls of past strategies and ensure that the UK’s life sciences sector delivers economic growth, high-skilled jobs, and better health for all. The launch signals not just a promise, but a commitment to sustained action, partnership, and progress throughout the next decade and beyond.


















