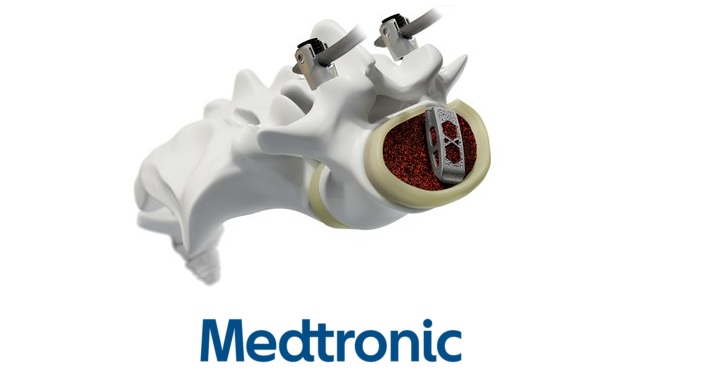
Medtronic plc , the global leader in medical technology, announced the U.S. launch of Adaptix™ Interbody System, the first navigated titanium implant with Titan nanoLOCK™ Surface Technology, a proprietary blend of surface textures on the macro, micro, and nano levels. The Adaptix Interbody System, mirrored after the veteran Capstone™ Spinal System, touts improved features for increased strength,1 subsidence resistance,1,2,3 easy insertion, and data-backed bone growth4,5. The announcement was made during the virtual edition of the North American Spine Society (NASS) annual meeting. Adaptix received U.S. FDA approval in August 2020.
This milestone represents the first 3D printed titanium implant, developed in house by Medtronic engineers, that incorporates the state-of-the-art Titan nanoLOCK Surface Technology.
Titan Spine, acquired in 2019, pioneered this surface technology that is the first to demonstrate the elements to be considered a nanotechnology for spinal devices as outlined in the FDA nanotechnology guidance document. Interbody implants are spacers that surgeons may insert between the vertebrae during spinal fusion surgery to help relieve pressure on nerves and hold the vertebrae in place while fusion occurs.
“Adaptix Interbody System allows me the best chance to meet my patients’ needs by confidently placing the implant under navigation and trusting the Titan nanoLOCK Surface Technology to allow the implant to promote fusion. Surface technology, material type, and implant design all play a role in bone growth process during fusion,” said J. Justin Seale, M.D. of OrthoArkansas Spine Institute. “The unique features and world-class technologies make the Adaptix Interbody System a truly differentiated implant.”
The Adaptix Interbody System addresses surgeons’ universal needs of fusion outcomes and offers:
- Trusted design with enhanced features.
- Science-backed nano surface technology.
- Navigation efficiency and confidence.
Medtronic continues to transform spine care and deliver on its Surgical Synergy strategy by offering solutions that integrate implants, biologics, and enabling technologies. Adaptix Interbody System is compatible with the Medtronic navigation platform (StealthStation™ Navigation and O-arm™ imaging) and the newly released Grafton™ DBF Inject, a unique graft delivery syringe that delivers an osteoinductive6 DBM into the surgical site.
“Adaptix Interbody System is an exciting addition to our portfolio that leads with our Titan nanoLOCK Surface Technology,” said Sharrolyn Josse, vice president and general manager of Medtronic Core Spine and Biologics division, which is part of the Restorative Therapies Group at Medtronic. “It is a fully navigated procedure, leveraging our leadership in navigation.”
About Medtronic
Medtronic plc, headquartered in Dublin, Ireland, is among the world’s largest medical technology, services and solutions companies – alleviating pain, restoring health and extending life for millions of people around the world. Medtronic employs more than 90,000 people worldwide, serving physicians, hospitals and patients in more than 150 countries.