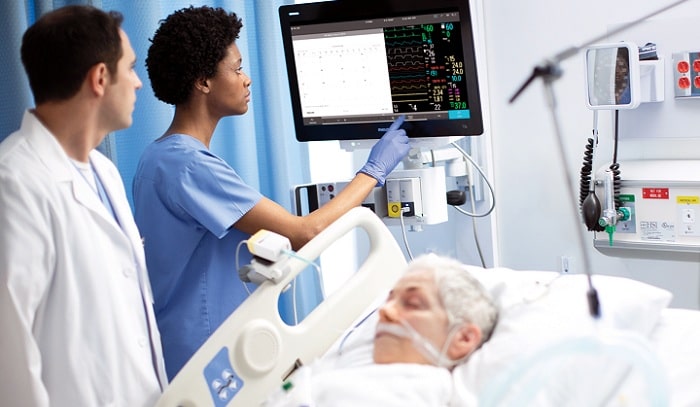
Royal Philips , a global leader in health technology, announced that its Philips Patient Monitors MX750 and MX850 have received 510(k) clearance from the U.S. Food and Drug Administration. Initially cleared for Emergency Use Authorization in 2020, the MX750 and MX850 are Philips’ most advanced patient monitors uniquely designed to support scalability, alarm management, cybersecurity and enhanced infection prevention within the hospital.
As health systems navigate the digital transformation, patient management solutions that can scale to meet hospital needs are in high demand. The MX750 and MX850 patient monitors are an integrated part of the company’s future-focused acute patient management solution, enabling hospitals to standardize at a system level and customize the solution in a particular care setting, even virtual or decentralized, to help optimize patient care. The monitors provide full modularity and are interoperable with other devices and applications, including Philips Patient Information Center iX and IntelliVue XDS software, to display critical patient data remotely or at the point of care.
“Continuous patient monitoring plays a vital role in overall patient safety while providing clinicians and caregivers with the holistic view they need to best support their patients and manage their workload,” said Sandra Lesenfants, General Manager, Hospital Patient Monitoring, Philips. “Philips Patient Monitors IntelliVue MX750 and MX850 are our most advanced patient monitors and a key part of our modular portfolio of hardware, software, services and consumables that can be tailored to provide integrated monitoring solutions that meet the individual needs of each healthcare provider.”
Data can also flow seamlessly between the MX750 and MX850 patient monitors and Philips Acute Care Telehealth command center to help to simplify clinical workflow with advanced clinical decision support, helping care teams better manage patient care across the continuum. The integrated system securely feeds patient data to a hospital’s electronic medical records via continuous, best in class, end-to-end encryption, meeting the industry’s evolving cybersecurity needs. The approach allows for virtually gap-free patient records from admission to discharge, even during transport, and connects care from the ICU to general ward, and across care settings.
This FDA 510(k) clearance allows Philips’ customers to expand their acute care and monitoring capabilities in the USA with the same MX750 and MX850 technology that has proven successful in hospitals across Europe since receiving CE mark in 2019.
“In the ICU, conditions can change quickly, so having access to real-time data is important to ensure signs of deterioration are not missed,” said Remko van den Akker, Registered Nurse, intensive care/critical care unit, Adrz Hospital, The Netherlands. “Philips’ solutions have helped us to improve the link between essential patient data and our hospital patient data management system, and allow us to automatically and securely share data. This level of interoperability has been key for successfully managing our acute care patients.”
Philips MX750 and MX850 patient monitors also feature a rugged design that can withstand the strongest hospital-grade disinfectants to help minimize hospital acquired infections without degrading the device. This helps caregivers to increase efficiency, while ensuring high quality patient care.
About Royal Philips
Royal Philips is a leading health technology company focused on improving people’s health and well-being, and enabling better outcomes across the health continuum – from healthy living and prevention, to diagnosis, treatment and home care.
Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips generated 2020 sales of EUR 17.3 billion and employs approximately 78,000 employees with sales and services in more than 100 countries.