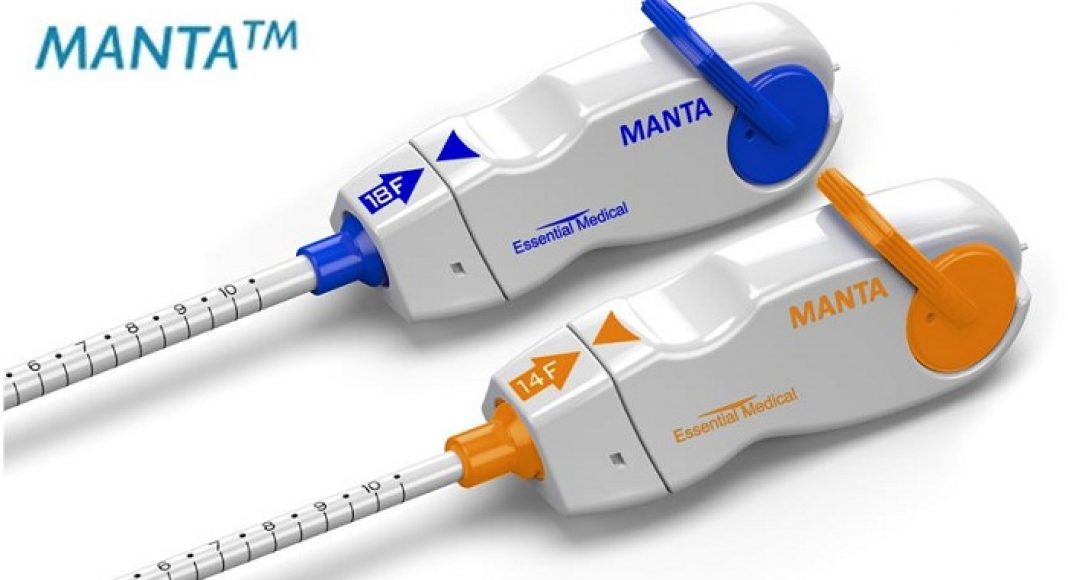
Teleflex Incorporated, a leading global provider of medical technologies for critical care and surgery, has announced that it received premarket approval (PMA) from the U.S. FDA for the MANTA™ Vascular Closure Device – the first commercially available biomechanical vascular closure device designed specifically for large bore femoral arterial access site closure.
The MANTA™ Vascular Closure Device is indicated for closure of femoral arterial access sites while reducing time to hemostasis following the use of 10-20F devices or sheaths (12-25F OD) in endovascular catheterization procedures.
The SAFE MANTA IDE Clinical Trial, the largest US prospective multi-center, single-arm trial of a purpose-designed large bore femoral access site closure, demonstrated that the MANTA™ Device successfully achieves fast reliable biomechanical closure with rapid hemostasis, with all primary and secondary endpoints met.1 With its innovative design, the MANTA™ Device has the potential to reduce bleeding complications and offset other procedural costs.1a
“I am very encouraged by the results of the SAFE MANTA IDE Clinical Trial. The clinically proven major complication rate (as defined by the study protocol) of 5.3% and VARC-2 Major Vascular Complications rate of 4.2% compare very favorably to suture mediated devices and the 24 second median time (65 second mean time) from deployment to hemostasis was impressive,” said Dr. Zvonomir Krajcer2, lead enroller and Co-Principal Investigator of the SAFE MANTA IDE Clinical Trial and Co-Director of the Peripheral Vascular Disease Service at Texas Heart Institute in Houston, Texas. “We have been patiently waiting for this approval, are eager to use the MANTA Device commercially and look forward to the efficiencies it can provide.”
“Our team has been working hard to obtain FDA premarket approval and were confident they would recognize the benefits that the MANTA™ Device can provide to the patient,” said Greg Walters, co-inventor of the MANTA™ Device and now Vice President of Access and Closure in the Interventional business unit of Teleflex. “We have had great success with the device in Europe over the last two years with over 10,000 units sold, and are thrilled to bring this innovative solution to patients in the U.S. and further fulfill this significant and previously unmet clinical need in the structural heart and endovascular space.”
With the MANTA™ Device, clinicians and hospitals can achieve:
Successful large bore closure with a device that is simple to use and does not require pre-closure, saving valuable time during the most delicate interventional procedures.
Low complication rates for fast reliable biomechanical closure with rapid hemostasis, potentially reducing costs.1a,b
Reproducible results, inspiring confidence in achieving successful closure.1c
“FDA premarket approval is another important milestone for the MANTA™ Vascular Closure Device,” said Stewart Strong, President and General Manager of the Interventional business unit of Teleflex. “Our commercial efforts in 2019 will include a measured launch of the MANTA™ Device to ensure strong initial outcomes with key thought leading physicians as we further invest in building the commercial infrastructure to support the long-term growth of MANTA™ Device revenues.”
About Teleflex Incorporated
Teleflex is a global provider of medical technologies designed to improve the health and quality of people’s lives. We apply purpose driven innovation – a relentless pursuit of identifying unmet clinical needs – to benefit patients and healthcare providers. Our portfolio is diverse, with solutions in the fields of vascular access, interventional cardiology and radiology, anesthesia, emergency medicine, surgical, urology and respiratory care. Teleflex employees worldwide are united in the understanding that what we do every day makes a difference.