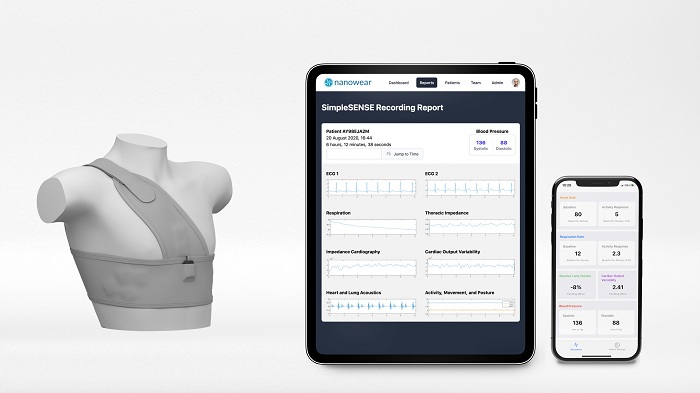
Nanowear has secured class II 510(k) clearance from the US Food and Drug Administration (FDA) for its SimpleSENSE cloth-based diagnostic platform.
Claimed to be the first-of-its-kind, SimpleSENSE is a multi-parameter remote diagnostic undergarment and machine learning digital platform that simultaneously and synchronously monitors and evaluates the heart, lungs, and upper vascular system.
The SimpleSENSE platform, which is gender-neutral and size adjustable, is developed to replace the digital stethoscope, multi-channel Holter monitor, Capnogram respiration machine.and blood pressure cuff.
SimpleSENSE enables to capture over 100 million data points per patient per day
SimpleSENSE serves as a diagnostic monitoring system to remotely capture over 100 million data points per patient per day across cardiac, pulmonary, and circulatory biomarkers.
With a rise in demand for telemedicine and remote diagnostic monitoring, SimpleSENSE offers a digital tool to evaluate medical data and trends between these biomarkers in an innovative way.
Nanowear intends to continue its SimpleSENSE clinical trials in diagnosing worsening heart failure and Covid-19, in addition to near-term commercialisation of SimpleSENSE platform.
Nanowear co-founder and CEO Venk Varadan said: “SimpleSENSE marks the company’s second FDA 510(k) clearance and follows Nanowear’s strategy of continued data-driven differentiation in the connected-care and remote diagnostic market.
“In the face of the unexpected and unprecedented Covid-19 public health emergency, Nanowear began working collaboratively with FDA to evaluate a broadened indication for use for SimpleSENSE.
“Our platform can now efficiently serve the new need for remote diagnostics across primary care, acute illness and procedure, and chronic disease cases.”
In July 2019, Nanowear commenced NanoSENSE heart failure management and alert diagnostic validation study.
Nanowear is involved in the development of patented and cloth-based nanosensor technology for use in different applications, including cardiac, neurological, and industrial safety.