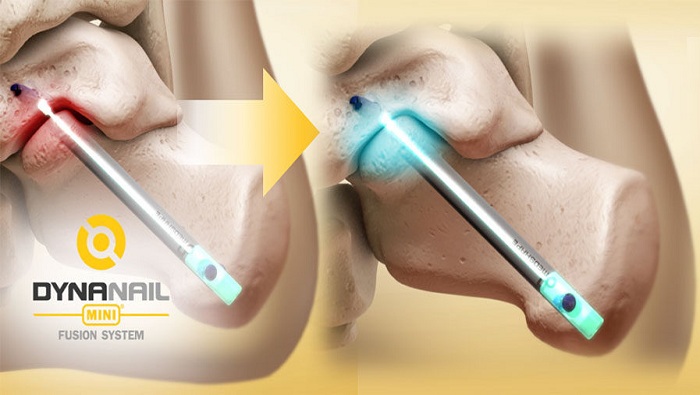
MedShape, Inc., the industry leader in orthopedic devices using advanced functional materials, announced the launch of the DynaNail MiniTM Fusion System. Featuring MedShape’s patented superelastic nickel titanium (NiTiNOL) technology, the DynaNail Mini is the first orthopaedic device designed specifically for subtalar fusion that offers maintained active compression post-surgery to promote healing and joint stability. The DynaNail Mini will be featured at the upcoming American Orthopaedic Foot & Ankle Society Annual Meeting in Chicago at the company’s booth #643.
The DynaNail Mini represents the latest innovation in the DynaNail product line to feature an internal NiTiNOL Compressive Element. NiTiNOL and its pseudoelastic properties have shown to be advantageous in applications where dynamic compression is required to facilitate healing of a fusion site.
While NiTiNOL has been used in orthopaedic surgery for over a decade primarily in bone staples, manufacturers have been challenged to machine NiTiNOL beyond simple two-dimensional geometries. MedShape engineers first revolutionized the use of NiTiNOL in alternate forms with the patented DynaNail® TTC Fusion System by incorporating an internal NiTiNOL component inside an intramedullary nail. Commercially available since 2013, the DynaNail has demonstrated successful outcomes in treating high-risk patient populations with challenging clinical scenarios with results reported in several peer-reviewed publications.1,2 The release of the DynaNail Mini signifies another milestone with the successful miniaturization of the internal NiTiNOL Element while still retaining the same dynamic compression performance, thus expanding the DynaNail brand to other applications.
Under a limited market release since earlier this year, the DynaNail Mini has been successfully implanted in a number of subtalar fusion procedures by leading foot & ankle surgeons across the country. Terrence Philbin, DO (Orthopedic Foot & Ankle, Columbus, OH) was one of the first users and has found the DynaNail Mini to be most useful in more complex cases.
According ot Dr. Philbin, “I was initially attracted to the DynaNail due to its excellent post-operative compression, particularly in patients with poor bone quality. From my experience so far, I have found the Mini nail to be easy to use and the results reproducible across my patient population.”
Naohiro Shibuya, DPM (Texas A&M College of Medicine) was also an early adopter of the new technology and shared similar sentiments: “There is finally an option to achieve dynamic compression in the foot other than staples. The DynaNail Mini nail allows long-term stability of the arthrodesis site without losing compression after bone resorption.”
During surgery, the Compressive Element is held in the stretched “activated” position and fixated with transverse screws in the talus and calcaneus. Post-surgery, the Compressive Element will automatically recover its stretched length in response to bone resorption or settling, allowing for compression to be maintained throughout the healing process. Manual compression can also be applied during surgery using the Mini Targeting Frame ensuring tight bone apposition immediately post-surgery. The carbon-fiber PEEK frame also features a robust, universal one-arm design allows for reliable drilling and screw placement. Unlike its predecessor, the DynaNail Mini comes packaged with the Compressive Element pre-stretched on a disposable Nail Guide that is attached to the Targeting Frame, thereby reducing the number of steps in the OR.
“As a market leader in NiTiNOL devices, we are proud to provide another novel solution that surgeons can add to their arsenal of surgical tools to address challenging pathologies,” said Kurt Jacobus, Chief Executive Officer of MedShape, Inc. “The early successful outcomes observed with the DynaNail Mini further validate the expansion of the DynaNail technology to other foot and ankle and trauma fusion applications. We are excited to see what the future holds.”
About MedShape, Inc.
MedShape, Inc. is a privately held medical device company working to develop and commercialize a portfolio of surgical solutions that uses its patented advanced functional material technologies to address the increasing demand for improved joint fusion, soft tissue repair and musculoskeletal trauma products in the foot & ankle.