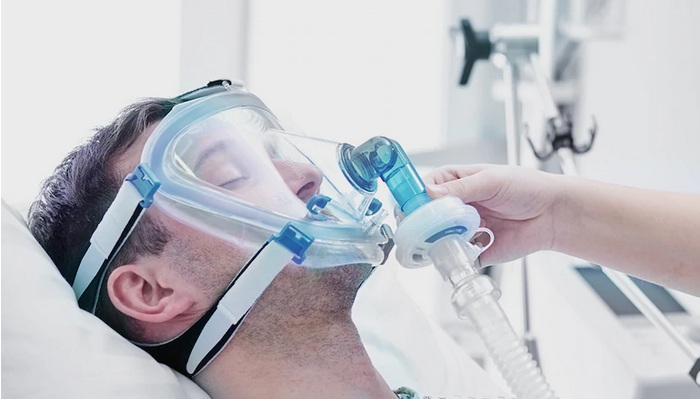
Nanotronics Health, LLC, a subsidiary of Nanotronics, announced that it has obtained EUA from the U.S. FDA for its non-invasive ventilator, nHale™, for at-home use. Nanotronics Health, LLC, also received authorization for the nHale™ to be used with supplemental oxygen under a doctor’s prescription.
“This approval provides flexibility for home care solutions and treatment, especially in the COVID-SARS-2 era,” said Dr. Mathew Foley, Vice Chairman of Northwell Health, New York’s largest healthcare provider serving NYC, Long Island, and Westchester.
As knowledge of the disease progresses, medical professionals and the public have become aware of how crucial non-invasive ventilation is for COVID-19 patient care in the hospital and beyond. To meet this need, Nanotronics Health, LLC, leveraged Nanotronics’ deep in-house expertise, incorporating advanced AI, Intelligent Factory Control (IFC) and sophisticated engineering to conceive, design and manufacture the noninvasive ventilator in under 90 days.
nHale™ is accessible to patients at a fraction of the cost of other noninvasive ventilators. With a one-button approach, the device is designed for quality, comfort, and ease of use.
“We wanted to provide hospitals and consumers with an affordable respiratory option to promote healing and aid the treatment of COVID-19,” said Julie Orlando, President of Nanotronics Health, LLC. “An innovative manufacturing vision was essential for building and scaling this critical device quickly—to get it in the hands of those who need it most.”
Nanotronics Health, LLC, developed and designed nHale™ to assist spontaneously breathing adults suffering from COVID-19 disease. It is intended for use in non-life-threatening situations, such as a patient in need of breathing assistance but not in need of invasive ventilatory support based on standard medical protocols.
About Nanotronics Health, LLC
Nanotronics Health, LLC is a subsidiary of and powered by Nanotronics. The company uses Intelligent Factory Control (IFC) to build and scale medical devices that are affordable, accessible and well-designed. Our first product, nHale™, a non-invasive ventilator, was conceived, built, and obtained Emergency Use Authorization by the FDA within 90 days to treat patients suffering from COVID-19. After obtaining Emergency Use Authorization, Nanotronics immediately began producing and shipping devices.
About nHale™
The nHale™ is a bi-level positive air pressure device to support respiratory therapy of spontaneously breathing adults weighing over 30kg suffering from COVID-19 disease. It is a non-invasive ventilator designed to be used in non-life-threatening situations, for spontaneously breathing patients, such as a patient in need of breathing assistance but not in need of invasive ventilatory support based on standard medical protocols.
The machine is for use in traditional healthcare facilities (e.g., hospitals, assisted living facilities, nursing homes) as well as spaces converted for the care of large numbers of COVID-19 patients (e.g., convention centers, university dormitories, motels, etc.). The nHale™ is also intended for use in home settings with a doctor’s prescription.
Supplemental oxygen may be used with the nHale™ device to increase the oxygen concentration of the airflow being delivered to the patient only when prescribed and trained by a qualified medical professional. The warnings must be observed when using supplemental oxygen with the nHale™ device.
Nanotronics Health LLC, a subsidiary of Nanotronics, applied Nanotronics’ deep in-house expertise, incorporating advanced AI, Intelligent Factory Control (IFC), and sophisticated engineering to build a machine that is easily manufactured at scale and at a reduced cost to increase accessibility for all Americans. The nHale™ device is designed for comfort and ease-of-use with the simplicity of one button.
nHale™ has been authorized by FDA under an Emergency Use Authorization [EUA]; nHale™ is authorized only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of the device; nHale™ has not been FDA cleared or approved.