Royal Philips, a global leader in health technology, and Vithas Group, Spain’s second largest private healthcare group, today signed a 5-year innovation and collaboration agreement in the areas of precision diagnosis and image-guided intervention. The agreement will allow Vithas Group hospitals and medical centers to benefit from the latest innovations in diagnostic imaging technology, healthcare informatics, and equipment for minimally-invasive interventional procedures. Vithas Group hospitals and clinics offer healthcare and advanced medical care in thirteen provinces in seven autonomous regions of Spain.
Under the terms of the agreement, Philips will deliver a technology management model that ensures Vithas Group’s current and future health technology needs in the relevant areas are met, together with the associated maintenance, updating, and equipment and system renewal needs. This management model will enable Vithas Group to achieve better results, provide optimal care at lower cost, and deliver a better experience for its patients and healthcare professionals.
In addition, Vithas will become a ‘reference technology partner’ for Philips in Spain, which means that its hospitals and medical centers will be able to implement new advances and innovations developed by Philips, before standard commercialization of these solutions in Spain.
This new alliance with Philips integrates cutting-edge technology, healthcare excellence, innovation, and research, which are the fundamental pillars on which we support our commitment to always seek the best experience for our patients,” said Dr. Pedro Rico, CEO of Vithas Group. “Furthermore, our professionals will have the most advanced technological solutions at all times, which will reinforce the quality of care and facilitate their research work.”
“We are very excited about the trust placed in Philips and convinced that, through this collaboration agreement, Vithas will have all the technology and innovation necessary to continue achieving the best health results and the best experience for its patients and its healthcare professionals,” said Juan Sanabria, President of Philips Ibérica. “We are proud to become the technological partner and strategic ally of a group as important as Vithas.”
The most advanced diagnostic imaging technology
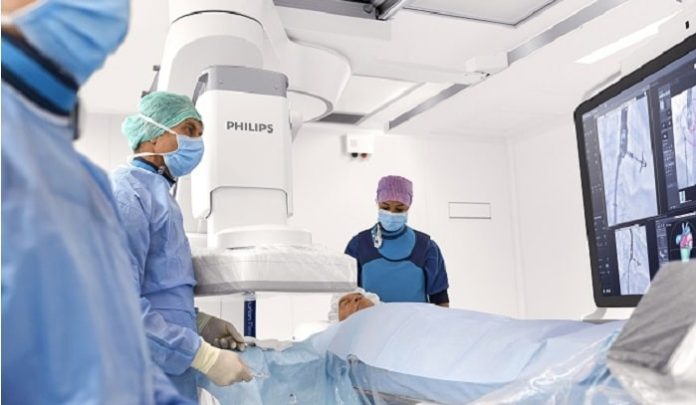
The agreement includes the replacement of Vithas Group’s existing MR imaging equipment with Philips’ latest innovative MR systems, which stand out for their image quality and patient comfort, and because they do not consume helium, also stand out for their sustainability. Philips will also install hybrid operating room solutions, including its latest ceiling mounted live-image guidance system for minimally-invasive interventional therapy, allowing Vithas hospitals to perform many types of procedure with maximum precision and safety. Four new hybrid operating rooms will be installed across four of the group’s hospitals.
Next to this, Philips will replace the Vithas hospitals’ CT (Computed Tomography) equipment by high-end equipment, allowing them to perform more precise cardiac diagnoses thanks to the Philips equipment’s speed, coverage, and high image quality, while also minimizing radiation dose. The agreement also includes X-ray equipment, solutions for dose management, and a Corporate Network for Diagnostic Imaging.
Benefits of the Corporate Network for Diagnostic Imaging
Through this network, and thanks to a solution for reviewing, post-processing and sharing information from advanced medical imaging studies, more than 100 radiologists will be able to work in a unified way with access to tools that speed up and improve the quality of results. Likewise, more than 5,000 referring physicians will have higher quality radiological reports directly available to them via a clinical viewer. The solution will also improve the patient experience, because they will be able to view their reports and images from any PC or mobile device via a secure web portal.
Joint scientific research
The innovation and collaboration agreement between Philips and Vithas Group will make it possible for the two organizations to collaborate in technological innovation projects in Vithas Group reference centers. These projects will involve joint scientific research in key healthcare areas, as well as the training of Vithas Group professionals in new technologies and clinical procedures.
About Royal Philips
Royal Philips is a leading health technology company focused on improving people’s health and well-being, and enabling better outcomes across the health continuum – from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips generated 2020 sales of EUR 19.5 billion and employs approximately 82,000 employees with sales and services in more than 100 countries.
About Vithas Group
Vithas’ commitment: accredited healthcare quality, personal service and long-term vision.
Vithas’ strategic commitment is that all healthcare is endorsed by the standards of the most prestigious international quality accreditation, the Joint Commission International. Only 15 prestigious hospitals in Spain have such accreditation and recognition, and three of them are part of Vithas, in Madrid, Malaga and Granada. Every year Vithas treats more than 5,200,000 patients in its 19 hospitals and 21 Vithas Salud medical centers. The 40 centers are located throughout Spain and include hospitals in Alicante, Almería, Benalmádena, Castellón, Granada, Las Palmas de Gran Canaria, Lleida, Madrid, Málaga, Seville, Tenerife, Vigo, Valencia and Vitoria-Gasteiz. The 26 Vithas Salud centers are located in Alicante, Elche, El Ejido, Fuengirola, Granada, Las Palmas de Gran Canaria, Lleida, Madrid, Malaga, Nerja, Pontevedra, Rincón de la Victoria, Sanxenxo, Seville, Torre del Mar, Torremolinos, Vilagarcía de Arousa, and Vitoria-Gasteiz. Additionally, Vithas has more than 300 locations throughout Spain as part of the Vithas Lab laboratories network. Its PlazaSalud24 purchasing center, a benchmark in the sector, serves 39 hospitals, 35 medical centers and 20 dental clinics.