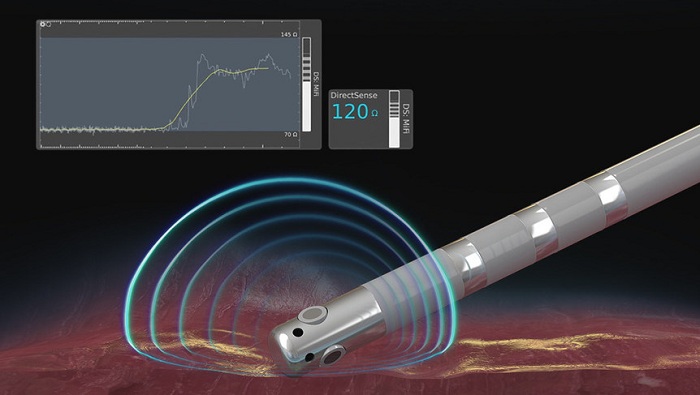
Boston Scientific announced the U.S. launch of the DIRECTSENSE Technology, a tool for monitoring the effect of radiofrequency (RF) energy delivery during cardiac ablation procedures. Available on the RHYTHMIA HDx™ Mapping System, the DIRECTSENSE Technology, which received U.S. FDA approval in April, is the only tool to monitor changes in local impedance – electrical resistance – around the tip of the INTELLANAV™ MiFi Open-Irrigated (OI) ablation catheter, offering physicians an additional measurement of therapy effect during an ablation.
Ablation is a treatment option for patients with cardiac arrhythmias in which physicians use a catheter to create lesions and destroy heart tissue that causes abnormal rhythms. The DIRECTSENSE Technology provides data on the impedance around the catheter tip to measure the ability of the tissue to respond to RF energy before physicians deliver therapy. During ablation, the tool tracks the change in local impedance which, in conjunction with other measures, offers physicians a distinct understanding of tissue characteristics and how they are affecting that tissue. These insights may indicate temperature change in the tissue, helping to reduce the chances of over-ablation and avoid complications.
“Knowing the change in impedance around the tip of the catheter provides unique information about local tissue characteristics and the development of the lesion,” said David J. Callans, M.D., professor of medicine at the Perelman School of Medicine, University of Pennsylvania – the first center to use the new technology in the U.S. “Unlike existing products on the market, the DIRECTSENSE Technology assists physicians in evaluating pre-ablation tissue health, while supporting better clinical understanding of how they are influencing that tissue to guide minimal, predictable ablation during a procedure.”
According to chronic data from the LOCALIZE clinical trial, a retrospective analysis of the DIRECTSENSE Technology presented by Ignacio Garcia-Bolao, M.D., director of cardiology and cardiovascular surgery, University of Navarra, Pamplona, Spain at Heart Rhythm Society 2020 Science, a local impedance decrease of ≥16.6 ohms with an inter-lesion spacing of ≤ 6mm showed a ≥ 98% positive predicative value of durable pulmonary vein block at three months in patients with paroxysmal atrial fibrillation (AF).
“Building upon the success seen with the DIRECTSENSE Technology in Europe, we are pleased to introduce this tool to physicians and their patients in the U.S.,” said Kenneth Stein, M.D., senior vice president and chief medical officer, Rhythm Management and Global Health Policy, Boston Scientific. “This approval marks an exciting milestone for our growing electrophysiology portfolio, providing physicians a more direct understanding of lesions and procedural efficiency to obtain optimal patient outcomes.”
The company continues to expand its electrophysiology offerings with the recently granted CE Mark for the POLARx™ Cryoablation System and plans to launch the product and begin enrolling European patients in a post-approval registry with the device in the coming months. The addition of this new single-shot therapy, alongside existing products and services, affirms the company’s commitment to providing meaningful advancements for the treatment of patients with AF.
About Boston Scientific
Boston Scientific transforms lives through innovative medical solutions that improve the health of patients around the world. As a global medical technology leader for 40 years, we advance science for life by providing a broad range of high performance solutions that address unmet patient needs and reduce the cost of healthcare.