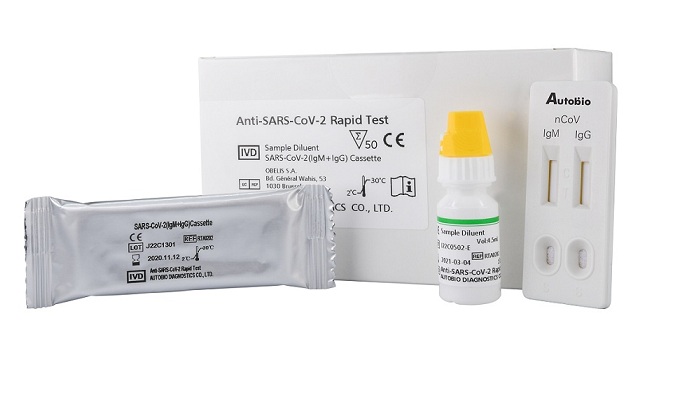
Hardy Diagnostics, a medical device manufacturer based inSanta Maria, California, announced FDA EUA approval on April24 for a new in vitro diagnostic medical device: Anti-SARS-CoV-2 Rapid Test (Cat. No. RTA0203).
This immunoassay is intended for qualitative detection and differentiation of IgM and IgG antibodiesto SARS-CoV-2 in human plasma or serum. This test was developed by Autobio Diagnostics Co., Ltd(603658, Shanghai), a publicly-traded, major microbiology medical device manufacturer, based inZhengzhou, China. Through this partnership, Hardy Diagnostics and Autobio have begun to opensupply chains to exclusively deliver this rapid test to the United States. This rapid market deploymentof a new in vitro diagnostic medical device was made possible through the FDA Emergency UseAuthorization (EUA) program. This program is used in times of crisis, such as the current COVID-19pandemic.
IgM antibodies are generated initially by the body as a result of infection at about the time symptomsappear. IgM antibodies will dissipate within approximately one month. IgG antibodies are generatedby the body about one week after symptoms appear and last for an extended amount of time.By using a patient’s serum or plasma specimen, the Anti-SARS-CoV-2 Rapid Test offers a turnaroundtime of only 15 minutes. This simple to use test requires no equipment or special expertise or trainingto implement.“We are incredibly proud of the work our partners in China have accomplished,” said Andre Hsiung,Director of Technical Services at Hardy Diagnostics.
“Because Autobio quickly developed thistechnology and because the FDA allowed Emergency Use Authorization, we will be able to moreeffectively leverage our sales network to get this product out to where it is needed the most.”The Anti-SARS-CoV-2 Rapid Test is a lateral flow immunoassay intended for the qualitative detectionand differentiation of IgM and IgG antibodies to SARS-CoV-2 in human plasma from anticoagulate blood (Heparin/ EDTA/ sodium citrate) or serum from individuals with signs and symptoms ofinfection who are suspected of COVID-19 infection. The Anti-SARS-CoV-2 Rapid Test is also intendedfor use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2,indicating recent or prior infection.Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of1988 (CLIA), 42 U.S.C 263a, to perform moderate or high complexity tests.
The Anti-SARS-CoV-2 RapidTest is intended for use by clinical laboratory personnel specifically instructed and trained in thetechniques of in vitro diagnostic procedures.Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in recentcontact with the virus, due to the lag time between exposure and the patient’s antibody response.
This test should not be used as the sole basis for patient management decisions. Test results must becombined with clinical observations, patient history, and epidemiological information.Follow-up testing with a molecular diagnostic test should be considered to confirm the infectionstatus. This test is not intended for the screening of donated blood.This test will be useful in identifying asymptomatic and mildly symptomatic carriers of the SARS-CoV-2virus. It will also be helpful in identifying persons who were infected by the virus previously, but maynot have been properly diagnosed.Studies suggest that antibody testing for COVID-19 may provide useful information in managing theinfected patient, making determinations as to immune status, and preventing the future spreading ofthe disease.
About Hardy Diagnostics:
Hardy Diagnostics is an FDA-licensed manufacturer of medical devices for microbiological testing withan ISO 13485 certified Quality Management System. The company manufactures over 2,700 productsfor the culture and identification of bacteria and fungi from its California and Ohio manufacturingfacilities. Hardy Diagnostics is headquartered in Santa Maria, California, and services over 10,000laboratories across the nation. In 2015, the company became 100% employee-owned. The companywas founded in 1980 by Jay Hardy, a Clinical Laboratory Scientist from Santa Barbara, California.Hardy Diagnostics maintains nine distribution centers nationwide and exports products to over 80foreign distributors. Hardy Diagnostics’ mission is to produce and distribute the finest products for thedetection of microorganisms and partner with its laboratory customers to diagnose and preventdisease.