Over the last decade, equivalent multi-modal diagnostic imaging technologies routinely used in most hospitals and specialist clinics have become established as a significant part of a researcher’s analytical toolbox in oncology.
A key part of preclinical research is the construction and testing of small animal models that allow scientists to gain a deeper understanding of human disease development and the efficacy and safety of potential treatments.
Today, the widely accepted goal to be able to offer patients more personalized cancer treatments is driving advances in preclinical oncology research – and imaging technologies, such as positron emission tomography (PET) have emerged as a vital tool in this work.
With numerous different types of tumors – including those not yet well characterized – and their varying responses to treatment, the search for new effective cancer therapies is incredibly challenging. Using technologies such as PET enables researchers to better understand the course of tumor progression and visualize cancer-related processes in real-time.
Importantly, by linking PET with other imaging modalities, such as computed tomography (CT) and single-photon emission computed tomography (SPECT), structural and functional imaging can both be generated in one experiment. So called ‘multi-modal PET/SPECT/CT systems’ are able to provide quantitative 3D images of radiotracers, bone, and soft tissue, furthering the growing knowledge of underlying cancer biology.
How it works
In simple terms, PET is able to picture metabolic processes in the body. It is able to provide information on the expression of receptors, energy metabolism, and other biomarkers of tumors, by imaging an intravenously injected radiotracer – a radioisotope, commonly fluorine-18 (18F), that is attached to a molecular probe targeting a specific molecule or metabolic pathway – and monitoring its uptake by tumor cells. In parallel, a CT scan makes use of computer-processed combinations of many X-ray measurements taken from different angles to produce cross-sectional images of specific areas of a scanned object, allowing the user to see inside the object without cutting it. The resulting co-registered PET/CT images provide essential knowledge at the boundary between research and clinical decision making.
Imaging insight
Much of the work being done in preclinical PET oncology research is concentrated in three key areas; understanding the biology of tumor development from molecular to organ level, predicting and monitoring responses to cancer treatments, and recognizing mechanisms of drug toxicity. Imaging technologies such as PET/CT have been successfully used in many areas of study. For example, it is well known that many cancers are associated with a higher metabolic turnover than normal cells so, using PET and an injected radiolabeled glucose analogue tracer, such as 18F-fludeoxyglucose (18F-FDG), glucose uptake can be quantified, and tumor burdens detected.
More specific PET agents that are capable of targeting the expression of one molecule or gene product have the potential to help researchers better understand and assess tumor biology and therapy responses. For example, the PET radiopharmaceutical 68Ga-PSMAhas revolutionized prostate cancer imaging in recent years. The Prostate-Specific Membrane Antigenfound in cell membranes is highly expressed in the prostate and in particular prostate cancer cells, making 68Ga-PSMA very effective in imaging.
In addition, PET/CT is used to determine the accumulation regions of 18F-FDG, to obtain a semiquantitative standardized uptake value (SUV) that assists in the diagnosis of tumor malignancy. Blood flow is another important marker, as tumor vascularization can potentially discriminate between nonneoplastic and neoplastic lesions.
Developing new imaging biomarkers
The development and characterization of imaging biomarkers to inform and guide cancer treatment management for individual patients is the focus of novel cancer therapy research. For example, the study of predictive imaging biomarkers and biomarkers that help to assess drug resistance and tumor response to drugs, as well as the development of specific imaging probes, could potentially guide innovative therapy plans and rapidly identify potential responders.
In one recent study, epidermal growth factor tyrosine kinase receptor (EGFR)-specific radioligands were used to measure EGFR expression in mice with head and neck squamous cell cancer (HNSCC). The aim was to define a predictive biomarker to stratify patients for treatment. Cetuximab is currently the only approved anti-EGFR monoclonal antibody(mAb) used for the treatment of HNSCC. This work is important because the ability to monitor and assess the drug’s efficacy and any cetuximab-mediated changes in receptor expression could help inform appropriate dosing with anti-EGFR antibodies.Data was acquired on a tri-modal small-animal PET/SPECT/CT system (Bruker Albira II, Bruker Biospin GmbH) and the results highlighted the potential for using EGFR imaging as a tool for assessing cetruximab efficacy based on receptor level, rather than relying purely on anatomical imaging, and provide image-guided therapeutic strategies for the clinic(figure 1) [2].
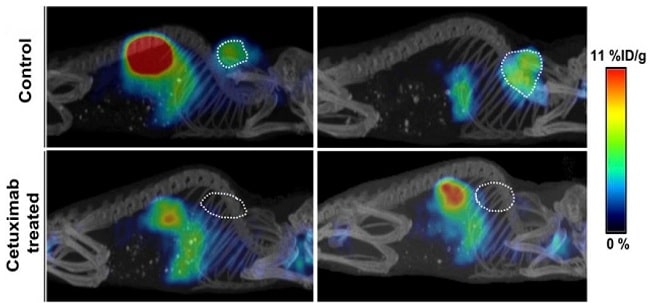
Figure 1: Representative sagittal whole-body PET/CT images of mice bearing HN5 tumors (outlined on image) with or without treatment with cetuximab. This research was originally published in JNM. Burley et al. J Nucl Med. 2019;60:353-361. © SNMMI and is reproduced in accordance with the Creative Commons Attribution License https://creativecommons.org/licenses/by/4.0/.
Investigating combination therapy
Cancers are often targeted with combination drug therapies. These regimens can address multiple molecular targets and reduce the chance of drug resistance. One study used preclinical PET/CT imaging to monitor 18F-FDG tumor uptake in an animal model for different treatment combinations: radiotherapy (Rad) alone, Rad + Temozolamide (Tmz), Rad + Mifepristone (Mife), and Rad + Mife + Tmz [3].
A multi-animal transport system on a tri-modal small-animal PET/SPECT/CT system(multi-
animal transport system – MATS – and Bruker Albira II, Bruker Biospin GmbH) was used to image the experimental subjects.
Rad+ Tmz is the typical treatment regimen for glioblastoma, but the study found that using Mife as a priming agent suppressed tumor growth more than the other treatment combinations (figure 2). The mechanism of this chemo-radio-sensitizing effect of Mife is yet to be fully characterized, but studies such as this help researchers make important steps towards improving available cancer treatments.
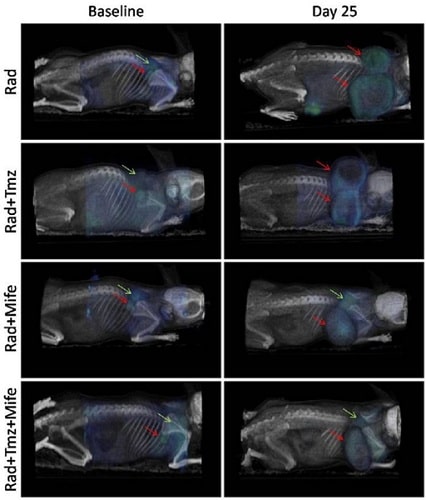
Figure 2: PET/CT images showing 18F-FDG tumor uptake, in four treatment combinations, at the beginning of treatment and 25 days later. Red arrows indicate tumor location at baseline and day 25, green arrows show sites of typical 18F-FDG uptake in brown adipose tissue (BAT). Reproduced from reference [3] in accordance with the Creative Commons License (https://creativecommons.org/licenses/by/2.0/).
Summary
Nuclear molecular imaging technology that has been established for clinical diagnostics is now being applied further back in the healthcare chain as researchers explore its benefits for investigating tumor biology and cancer treatment. The ongoing development of multi-modal PET technology specifically for preclinical applications looks set to continue this drive, and the benefits are already changing the way cancer is treated, moving towards the goal of a more personalized medicine approach.
About the Author
Todd Sasser, Ph.D is a Head of Applications for Bruker Preclinical Imaging. Dr Sasser studied at The University of Liverpool and The University of Hawaii and was a visiting scholar at The University of Notre Dame.
References
1. Jones T and Townsend D (2017) History and future technical innovation in positron emission tomography. J Med Imaging (Bellingham). 4(1):011013. doi:10.1117/1.JMI.4.1.011013.
2. Burley TA, Pieve CD, Martins CD, Ciobota DM, Allott L, O WJG, Harrington KJ, Smith G and Kramer-Marek G (2019) Affibody-Based PET Imaging to Guide EGFR-Targeted Cancer Therapy in Head and Neck Squamous Cell Cancer Models, J Nucl Med, 60:353-361.
3. Llaguno-Munive M, Medina LA, Jurado R, Romero-Piña M, Garcia-Lopez P (2013) Mifepristone improves chemo-radiation response in glioblastoma xenografts. Cancer Cell International. 13:29. https://doi.org/10.1186/1475-2867-13-29.
For more information on Bruker’s PET/CT imaging solutions for preclinical oncology research, please visit https://www.bruker.com/applications/preclinical-imaging/oncology.html.