SCC is the leading and most widely recognized provider of integrated information systems for the healthcare industry.┬Ā A privately-held organization, SCC reinvests a large percentage of net revenue annually (generally around 20 percent) towards R&D and our LIS.┬Ā With nearly forty years of LIS experience, SCC has a diverse global workforce of nearly 2,000 information┬Ātechnology, medical technology, communications, and business professionals.
SCC is the worldŌĆÖs largest LIS/LIMS software developer with 1,620 professionals dedicated to LIS design, development, delivery, implementation, and support.┬Ā Our robust of fully integrated laboratory and genetics information management systems provide healthcare clients the flexibility and scalability to be competitive.
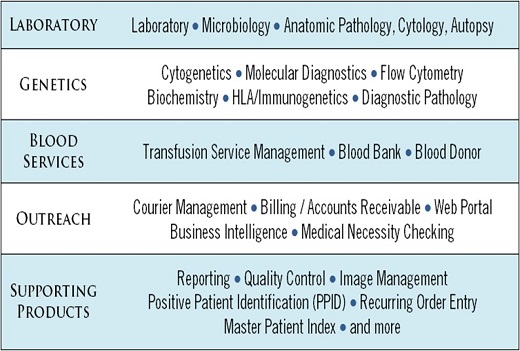
SCC offers a greater depth and breadth of laboratory modules than any other vendor.┬Ā A ŌĆ£one-stop-shopŌĆØ healthcare IT vendor, SCC offers multiple disciplines in one integrated system so clients can rely on a single vendor for their entire integrated system instead of choosing a vendor for each.┬Ā SCCŌĆÖs innovative and complete solutions are setting the standard for healthcare IT with suites of fully integrated information management systems.
Recognizing that clients have different needs, we offer optional productivity modules.┬Ā We do not force our clients to purchase software modules they do not need.Considered a leader in the LIS field, SCC roduces the most robustŌĆöand most automatedŌĆöLIS on the market.┬Ā With the SoftLab LIS, laboratories require fewer laboratory FTEs and are able to process added volumes without adding FTEs.┬Ā SCCŌĆÖs laboratory and genetics information system include solutions for these healthcare disciplines and more
Delivering integration and interfaces in complex environments
The complex intricacies of clinical interfacing is a job best left to the experts.┬Ā SCC Soft Computer has an unmatched reputation for writing complex interfaces and working with our industry colleagues to ensure that our clientsŌĆÖ clinical environments get the data exchange they need.┬Ā We understand that interoperability between clinical applications is critical for success whether it is our own robust healthcare information technology solutions, an instrument, or a module from another vendor.
With a proven record for accomplishing complex LIS implementations in large, multisite environments, SCC excels in delivering complex interfaces and integration projects.┬Ā SCC outperforms the competition with our best of breed approach to integration projects.┬Ā Our laboratory and genetics information system solutions provide robust functionality, ease of instrument interfacing including robotics, Web-enabled tools , rules-based logic, multisite capabilities, and more.┬Ā SCCŌĆÖs fully integrated systems provide a seamless interface that links all clinical laboratory departments throughout the care provider network.
All SCC systems are designed and developed by SCC architects and programmers; all modules are integrated and run on a single database instance producing consolidated reports.┬Ā Our systems share a platform of architectural ŌĆścommonsŌĆÖ that provides the basis of this integration.┬Ā Increased consistency between systems, timely delivery of test results, flexible order entry with real-time results reporting, automatic workflow, safety features that are second to none, long-term cost savings, and interoperability oupled with the ability to exchange information between systems are just a few of the many powerful features built into SCCŌĆÖs robust healthcare information systems.
As a best-of-breed LIS, SoftLab has always been ŌĆśopenŌĆÖ, and SCC is implemented in the most complex environments (multisite, multi-time-zone/global, all lab disciplines, billing).┬Ā With SCCŌĆÖs advanced system architecture, we offer many ways to integrate with instruments and foreign systems.┬Ā Our interfacing team has developed robust direct interfaces, which eliminate the need for middleware/data manager, reduce costs, and decrease the complexity of the installation and implementation.
SCCŌĆÖs SoftLab┬« LIS is a multi-threaded application, so a technologist can place and view multiple instruments on a single SoftLab LIS from a single workstation.┬Ā This is important because SoftLab also has a powerful rules engine and is a rules-based system.┬Ā By contrast, other vendor systems must rely on a data manager for rules and QC.┬Ā As a result, rules and auto-verification enable a single tech to do the work of several using the SoftLab LIS.┬Ā During third shifts, a single tech could run the entire lab from a single workstation.┬Ā These LEAN/Six Sigma-type processes cannot occur without direct interfacing to instruments and without the rules engine.
Integration of laboratory systems is a key quality for successful lab management, and interoperability between clinical applications is critical for success.┬Ā We have a history of accomplishing complex LIS implementations in large, multisite environments and offer a greater depth and breadth of laboratory modules than any other vendor.┬Ā We produce the most robust and most automated LIS in the world.┬Ā With our SoftLab LIS, the clinical laboratory can process added volumes without increasing staff.┬Ā And as a functionality richer solution, SCCŌĆÖs rules-based laboratory and genetics information systems provide specialized efficiency tools within each laboratory discipline without the need for additional third-party systems.
SCC is committed to providing integrated products to our clients to ensure seamless and efficient management of their diverse laboratories.┬Ā In keeping with our core value of integration, we have several ongoing integration initiatives between our laboratory and genetics information system applications.┬Ā For example, SCCŌĆÖs Genetics Information Systems Suite┬« is our fully integrated set of genetics information management systems covering a wide range of specialized testing.
Although these systems were designed, built, and function as a single integrated solution, the individual modules can function as standalones or in any combination that clients can purchase to best suit their test offerings.
SCCŌĆÖs integrated solutions enable clients to adaptŌĆöallowing them to provide better services to their patients and decrease their operational and IT footprints while increasing opportunities for expansion and revenue-building.┬Ā The flexibility to evolve with these changes and respond in a timely manner is one of the key differentiators that sets SCC Soft Computer apart.
Managing TCO for maximum ROI
SCC is committed to managing our clientsŌĆÖ total cost of ownership or TCO.┬Ā We believe software TCO is established long before we write a single line of code, and we consider this during the design process when the objectives of the software relative to installation/implementation, user requirements, functionality, and ease-of-useŌĆöare determined.┬Ā This is the foundation of SCCŌĆÖs software development lifecycle.┬Ā With this in mind, we begin our design/development and TCO management process with these three principles/standards:
- Develop systems with ease of use
- Manage costs over the lifetime of the software
- Build data migration and integration into the product
Installation, setup, configuration, training, and ease-of-use will ultimately impact a clientŌĆÖs TCO in a far greater way than will the initial cost of purchase, and our clients participate in the design of our systems.┬Ā SCC develops our laboratory and genetics software based in large part on user requirements gathered by our network of subject matter expertsŌĆöallied healthcare and healthcare IT professionals themselvesŌĆöwho work directly with our clients.┬Ā Common words and terminology used in the clinical laboratory environment by knowledgeable, trained, and experienced personnel create a familiar, friendly user interface.┬Ā This development practice combined with our experience and expertiseŌĆöhas placed SCC at the forefront of laboratory, genetics, blood services, and outreach information systems software development.
c when a proper workflow assessment and analysis has not been performed, the new system tends to not yield the expected benefits.┬Ā As varied as the reasons are for purchasing a new clinical system, the reasons for implementing change are quite simple:┬Ā to automate the processes necessary to meet all business goals with an eye on future growth.
A TCO analysis is critical in helping to ensure that all costs are considered.┬Ā Leveraging our nearly forty years of experience designing, developing, delivering, implementing, and supporting information management solutions for the clinical laboratory environment, SCC helps clients analyze their workflows to perform unbiased assessments of their current usage of their LIS functionalityŌĆöand makes recommendations on how they can optimize their systems.
┬Ā ┬Ā ┬Ā ┬Ā ┬Ā ┬ĀSCC by the numbers
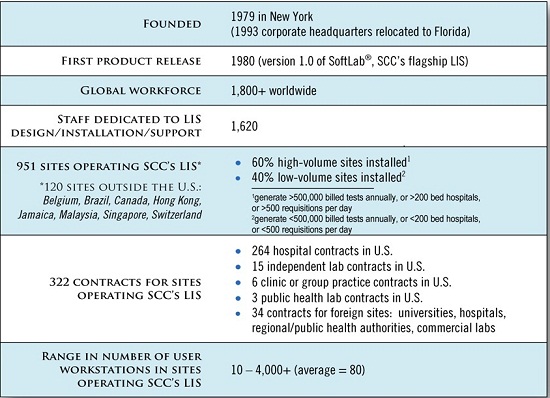
SCC receives phenomenal feedback from our client base and the independent users group, SNUG (Soft Network Users Group).┬Ā Through the processes of SCCŌĆÖs software development lifecycle, this client feedback becomes incorporated into future releases.
Contract wins in the past several years at many of the worldŌĆÖs most renowned academic medical institutions highlight our success and leading position within the global LIS market
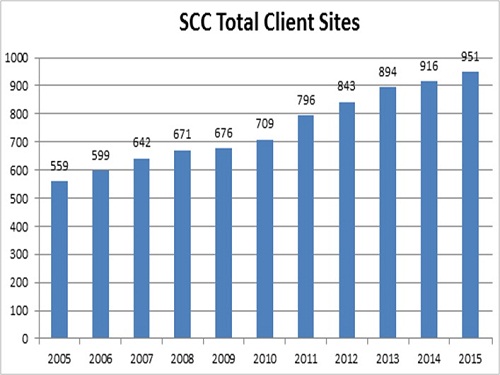
This dedication to R&D protects our clientsŌĆÖ investment in our LIS, and ensures that our systems will provide the greatest levels of automation, economy, and patient safety available from any vendor.
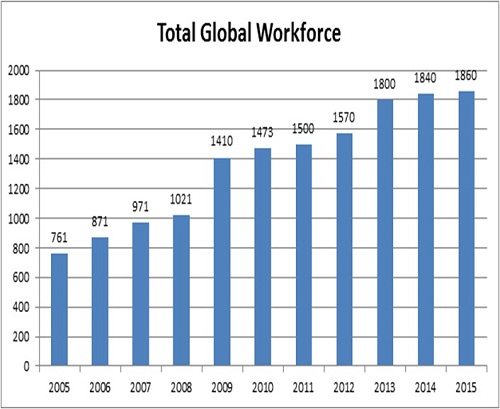
With development staff of well over 1,000 worldwide, SCC services some of the most demanding laboratory environments, and innovation is essential.
Since the companyŌĆÖs inception in 1979, SCC has avoided layoffs and has continued to add positions and new career paths.┬Ā The company is determined to stay on this course.┬Ā Our average rate of turnover is consistently at least 40% lower than the industry standard.




















