Point-of-Care testing may be considered essential for patient care in emergencies where time is of the essence and delays in diagnosis and stabilisation mean the difference between life, death or dysfunction.
A rapid understanding and correction of physiological imbalances supports quick decision making in emergency settings and critical care units, such as adult and neonatal intensive care environments. A slight deviation in biochemical and haematological markers can pin point decompensation or recovery.
Along with various “vital signs” such as blood pressure, heart rate and rhythm; temperature and respiratory rate; and some biochemical markers reflect the rapid changes that occur when a patient becomes unstable.Given that “time is life,” having the ability to test, diagnose and treat improves patient care. Point-of-Care (POC) testing may improve a patient’s outcome by allowing real-time detection and subsequent treatment of the physiological deterioration.
 Studies show that POC testing have the advantage of providing reduced therapeutic turnaround time, shorter door-to-clinical-decision time, rapid data availability, reduced pre-analytic and post-analytic testing errors.1,2 At the same time, leaps in technology have made POC instrumentation compact, portable, self-contained,user-friendly and needing only small sample volumes. Key to the success and adoption of POC systems is knowledge of their irrevocable reliability and evidence that POC may be an equally effective substitute for existing laboratory systems.
Studies show that POC testing have the advantage of providing reduced therapeutic turnaround time, shorter door-to-clinical-decision time, rapid data availability, reduced pre-analytic and post-analytic testing errors.1,2 At the same time, leaps in technology have made POC instrumentation compact, portable, self-contained,user-friendly and needing only small sample volumes. Key to the success and adoption of POC systems is knowledge of their irrevocable reliability and evidence that POC may be an equally effective substitute for existing laboratory systems.
Ensuring reliability requires equipment that is tried and tested, accurate and precise, not only for day-to-day requirements but also for maintaining users’ trust where there is very little room for error. The equipment supplier, supporting technicians, technologists and nursing staff all need to buy in to the benefits of the diagnostic aid so as to maintain an unwavering commitment to drive and support quality management systems (QMS) based on good laboratory practices.
Investing in quality management systems to support blood gas POC testing at Life Healthcare facilities
Life Healthcare operates 140 Siemens RAPIDPoint500 blood gas machines in 42 hospitals. The blood gas analysers are located in casualties and ICUs (adult and neonatal). Blood gas systems in hospitals have traditionally been supported by laboratory technicians, while the use of diagnostic equipment and patient testing have been performed by nursing teams in the clinical setting. Due to the rising costs of pathology,Life Healthcare, with the support of specialists, took over the management of the blood gas analysers so as to reduce these costs and offer more affordable patient care. To maintain the commitment of providing quality, Life Healthcare has undertaken to ensure that Life Blood Gas Services aligns with SANAS requirements for Good Laboratory Practice (GLP).
GLP standards are required for laboratories, but not necessarily POC devices. . With the assistance of a Pathology Consultant, a unique Life Healthcare POC Quality Management System was developed to meet GLP requirements, including ISO9000 and subsequent ISO22870 and ISO15189.
This included investing in consulting pathologists, medical technologists, IT platforms that monitor the functioning of every blood gas analyser in real time, formal training and certification of nursing staff operating the equipment, clinical engineers and internal and external quality assurance. To date, Life Healthcare in association with an international external quality assurer have achieved 100% certification in external quality assurance when bench marked with approximately 140 local and 64 international laboratories conducting blood gas testing.
Quality Challenge – How does Life Healthcare’s blood gas system compare with an external laboratory analyser?
In keeping with the commitment to promote POC adoption and further drive specialist and nursing trust in the blood gas system, Siemens, the supplier of the Siemens RAPIDPoint500 was challenged on the reliability of their equipment.
At the end of 2016, Life Healthcare undertook a comparative analysis at Life Eugene Marais Hospital of results from the Siemens RAPIDPoint500 and the Gem Premier 3000 (GemPrem), provided by a private laboratory. The aim was to compare at least 40 blood gas tests performed in the neonatal and adult ICU’s over a period of three months.
The project was introduced by Dr Riyas Fadal, National Complementary Services Manager and the technical evaluation was performed by consulting pathologist Dr Shahed Omar, a specialist chemical pathologist and ICU intensivist.
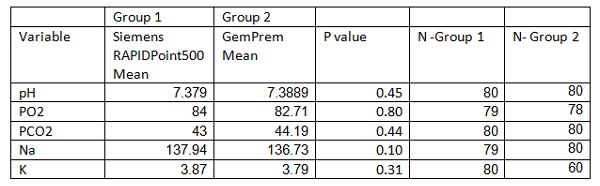
A comparison of the following measured variables on the two blood gas systems was done: pH, partial pressure of carbon dioxide in mmHg (PC02), partial pressure of oxygen in mmHg (P02), glucose in mmol/l and lactate in mmol/l. In addition they compared the Sodium (Na) and Potassium (K) values (both in mmol/l) obtained on SiemensRAPIDPoint500 to the laboratory measured serum Na and K values which were considered as the reference. The blood sample for each of the measurements was collected by the same person. The blood gas analysis was performed within 5 minutes of each other.
Results
Technical feasibility analysis revealed that the Siemens RAPIDPoint500 analyser had an error rate of less than 1% compared to the 7.2% error rate produced by standard laboratory blood gas systems. The overall number of test failures on the Siemens RAPIDPoint500 analyser was only 3 out of a total of 320 tests (80 samples x 4 analytes).
Using the Bland Altman Plot, a good correlation with larger laboratory systems was demonstrated for specific analytes.
Specialists in the field of neonatology had initially raised concerns about abnormal low readings for specific analytes that are sometimes found on blood gas systems. To assess this and better understand the equipment’s limitation, the blood gas Na and K results of the Siemens RAPIDPoint500 were compared with results from a larger laboratory system. Results were found to be in agreement with each other.
No significant differences were noted between any of the tests in table 1. The two blood gas instruments therefore provided statistically equivalent results for the above tests.
Differences between normally distributed parameters using the T Test for independent samples
Our authors
Dr Shahed Omar
Dr Shahed Omar is registered as a specialist chemical pathologist with the Health Professions Council of South Africa (HPCSA) with 15 years of experience in Critical Care and a Diploma in Anaesthetics. He has been an investigator for several clinical trials, been involved in an accreditation process and played a major role in the start-up of a new clinical pathological service, just to name a few. Furthermore, he also presents training sessions on the Sub-Speciality of Critical Care to his fellow registrars.
Claudette Kruger
Claudette Kruger holds the role of Point-of-Care Standards and Support Specialist at Life Healthcare and is a registered Medical Technologist with a BTech in Biomedical Technology. She has 9 years clinical laboratory experience as well as management and development of QMS systems for medical laboratories and POC diagnostics.
References:
1. Kapoor D, Srivastava M, Singh P. point of care blood gases with electrolytes and lactates in adult emergencies. Int J Crit Illn Inj Sci. 2014 Jul-Sep; 4(3):216-222
2. Louie R, Tang Z, Shelby D et al. Point-of-care testing: Millenium technology for critical care. Lab Med. 2000; 31(7): 402-408




















