Introduction
Americans spend about $3 trillion per year on healthcare, or about $10,000 per person per year. Despite these expenditures, Americans are worse off than their international counterparts with respect to infant mortality, life expectancy and the prevalence of chronic conditions.
Most public policy debates and legislation focus on how to recover these costs, which invariably ends in a null set.
This paper proposes a new standards-based approach for fixing the inefficiencies plaguing the healthcare industry in the United States. As described herein, a non-profit standards body would be established by Congress to bring a coordinated approach to healthcare for each of the top ten chronic diseases.
Such an approach would establish consistent priorities and practices across all of the components of the healthcare industry affecting these chronic diseases, including standards of care, areas of research emphasis and insurance guidelines.
Under such an industry structure, patient care would improve and the overall costs for the provision of healthcare would drop precipitously.
The U.S. Healthcare Market Doesn’t Function Effectively or Efficiently
Looking at the components of the healthcare industry from standards of care to prices to insurance coverage to potential new treatments, virtually none of the relevant choices are understood by the consumer. What this means from an economic point of view is that there is no “invisible hand” of the marketplace driving an effective and efficient outcome from the interplay of supply and demand.
Equally troubling, none of components of the overall industry work in lock-step. For example, the FDA could have indirectly required billions of dollars of research to be spent on a drug before it gives its approval, only to find that the organizations that provide guidelines for care don’t promote its use and the insurance carriers don’t contribute towards its charges.
One way to think about the current system is depicted in the following chart:
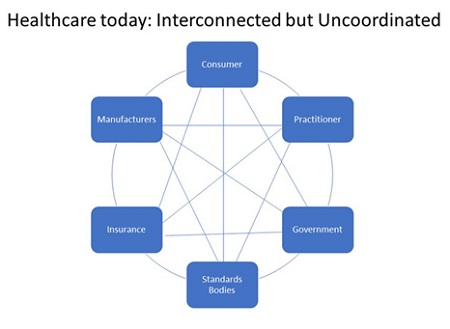
As the chart implies, all of the components of the healthcare industry impact each other. However, they don’t work in lock-step to achieve a certain outcome. Moreover, the consumer is hard-pressed to make heads or tails of the best approach for his or her condition given that the relevant information for each component is hard to get. This is particularly true for chronic illnesses. Hence, the current system almost guarantees a misallocation of resources
In broad terms, the cost of healthcare in the United States is approximately $3.0 trillion per year (or $10,000/person), including:
• 32% for hospital care
• 20% for physician and clinical services
• 10% for prescription drugs
• 5% for nursing care facilities and continuing care retirement communities
When looking for causation for these healthcare expenditures, there are many large targets including heart disease, diabetes, asthma, cancer, Alzheimer’s, Parkinson’s and Epilepsy.
Taken together, chronic diseases cause 7 of every 10 deaths in America. Moreover, about 25% of people with chronic diseases have some type of activity limitation such as needing help with dressing or bathing or being restricted from work or attending school.
By any measure, chronic diseases take a heavy toll on the nation’s health and wealth. For example, heart disease and stroke are estimated to cost over $430 billion per year. Diabetes costs are estimated to be about $175 billion per year. Alzheimer’s is estimated to add about $150 billion per year. And cancer adds over $200 billion per year. In total, over 50% of the $3 trillion spent on health care each year is attributable to chronic diseases.
The logical question to ask is: “what is being done about it?” The answer is a quite a lot, but not very effectively or efficiently.
The FDA has been at the center of the process of new healthcare solutions for over a century.
The FDA has done a good job in achieving its legislative charter of ensuring that drugs and devices are safe and effective. For example, over the last 50 years, only 2% of all FDA-approved drugs have been taken off the market to due to safety concerns.
In looking at the government website for clinical trials, it lists over 270,000 research studies. However, no one prioritizes this research for any particular objective. Moreover, there is no coordination on which promising research should be pursued for which illnesses. The sad reality is that most of these studies don’t produce meaningful benefits for people.
One important reason for the lack of progress is that the FDA has defined a process where a drug takes 12 years on average to be approved at an average cost of over $1 billion. These estimates do not include the FDA’s impact on preclinical research, which can add more than a decade to the process and additional costs.
The impact and cost of delay from the FDA approval process is large, not only from the enormous costs of treatment but from premature death of those losing out on new treatments. For example, in 2012, I filed a Citizen’s Petition with the FDA for it to withdraw support of ACT chemotherapy for BRCA1-related breast cancer and enable women to be treated with a PARP inhibitor and a platinum-based drug. The Petition was denied. However, this past year, the FDA approved such an approach. In the interim period, thousands of women died prematurely from the inferior treatment afforded by an antiquated standard of care.
Announcing a desire for cures is not enough. For example, it has been more than 50 years since President Nixon declared a “War on Cancer”. More recently, President Obama announced a major initiative to seek cures through precision medicine. However, when I subsequently petitioned the FDA to issue a Notice of Proposed Rulemaking seeking input on how best to analyze and approve, as appropriate, new Precision Medicine Initiatives, the FDA responded by saying it “has been unable to reach a decision on your petition due to the need to address other Agency Priorities”.
It is a lack of action on important matters such as this that led the former Director of the National Cancer Institute, Vincent T. DeVita, Jr. M.D., in The Death of Cancer (2015) to recommend that the FDA should not have approval authority over Phase 1 and Phase 2 clinical trials for cancer (and, by inference, should not participate in regulating preclinical cancer research).
After all of its internal efforts to improve its procedures, the FDA’s current processes still leads to only 20 or 30 drug approvals per year, and much fewer blockbuster remedies.
The FDA effectively acts as a barrier to competitive entry, thereby ensuring excessive profits for the pharmaceutical companies.
Beacons of Hope
Michael J. Fox was diagnosed with Parkinson’s disease in 1991 at the age of 29. After observing the progress, or lack thereof, on Parkinson’s disease, he established the Michael J. Fox Foundation for Parkinson’s disease (MJFF) to develop a comprehensive approach to the disease and to find a cure.
Since 2000, MJFF has funded more than $750 million to pursue a cure for Parkinson’s disease. It is collaborating with literally scores of organizations with respect to funding, in-kind contributions, strategic input and research initiatives. It also provides extensive information to the public at no cost, including weekly video briefings, covering issues such as diagnosis and symptoms, living with Parkinson’s disease, treatment options and promising new research.
Another example of an organization trying to coordinate an approach to a disease is the National Comprehensive Cancer Network® (NCCN®), which is a not-for-profit alliance of twenty-eight leading cancer centers devoted to patient care, research, and education. The NCCN® is dedicated to improving and facilitating quality, effective, efficient, and accessible cancer care so patients can live better lives. NCCN® programs offer access to expert physicians, superior treatment, and quality and safety initiatives that continuously improve the effectiveness and efficiency of cancer care globally.
Despite these bright spots, the current healthcare system doesn’t work efficiently because the components generally are not connected or coordinated by the “invisible hand” of the marketplace. This market failure must be addressed to improve healthcare in the United States
An Integrated Approach for the Healthcare of Chronic Illnesses
Just as the private market has failed, it is not reasonable to assume that the federal government can somehow orchestrate an efficient and effective outcome, as Hillary Clinton once tried to pursue during her husband’s presidency. At a minimum, such an approach would be subject to political instability, as witnessed with the Affordable Care Act.
Rather, the best approach would be to build on successful models in practice today and establish an industry-wide organization with standards-setting responsibilities. That is, Congress would set up a non-profit quasi-governmental standards body to establish the scope and direction of care for chronic diseases.
The following chart shows how the healthcare industry should be organized to improve care and lower costs for chronic illnesses.
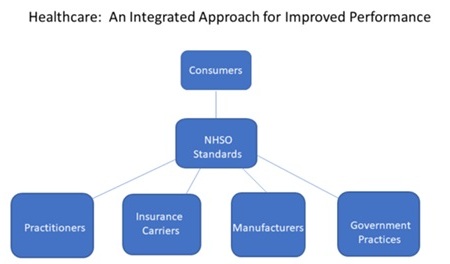
The first steps for the newly-established NHSO would be to establish standards of care for each of the Top 10 illnesses and to develop short-term and long-term objectives based upon the industry resources dedicated to these illnesses. These objectives could include the avoidance of the illness in the first place from vaccines or other measures.
Secondly, the chapters would define the interfaces and best practices for the various components of the industry affecting their relevant patient populations.
For example, each chapter would establish the benchmark for the basic level of insurance coverage for various procedures and treatments based on the approved standard of care. They would also prioritize the efforts for specific research initiatives. Equally important, they would establish the informational, pre-clinical and clinical requirements most appropriate for each chronic illness.
The ultimate value of the NHSO and its chapters would be to create a better functioning private market for each of the Top 10 chronic illness markets in the United States.
Setting Standards
Governments worldwide have recognized the importance and impact of standards on their economies. Standards have significant procompetitive effects such as increasing price competition as standard products are more readily compared and solving issues such as product compatibility and consumer safety.
Standards bodies can be governmental, quasi-governmental or non-governmental. Quasi-governmental and non-government standards bodies are often non-profit. Quasi-governmental organizations are supported by the government but managed privately.
In the healthcare industry, we know that there is a need for:
• the consumer’s voice to be heard and a set of objectives to be set to address those voices;
• a standard of care to be developed and communicated for the target populations; and
• for all of the components in the industry to address the consumers’ requirements on a coordinated and efficient basis.
To help achieve these objectives, a non-profit, quasi-government standards organization called the National Healthcare Standards Organization (NHSO) should be established to publish guidelines and coordinate the practices of the industry as follows:
1. The NSHO would be created by Congress with separate chapters for each of the Top 10 chronic diseases.
2. Each chapter would have the same charter: namely: 1) to represent the patients afflicted with a specific chronic illness and 2) determine the preferred standard of care in detail for its particular illness. This would include standards for the prevention, diagnosis and management across the continuum of care.
3. The practices and prescribed treatments of the preferred standard of care would, in turn, be codified as qualifying for mandatory payments to be made by insurance companies.
4. Each chapter would establish short-term and long-term objectives for their patient population based on available industry resources.
5. Each chapter would set the priorities for existing Research and Development projects and identify promising areas for additional funding.
6. Each chapter would determine the appropriate FDA approval process for the informational, device and drug applications in its field. That is, the FDA review process could be streamlined for a particular application without the need for legislation. Final approval authority for commercial release would rest with the FDA.
The NHSO and each chapter would have five representatives including medical experts in the relevant field and a member of the public. The composition of the Chapter Members should represent the core medical specialties and clinical expertise involved in the specific standard of care (with exclusions for any conflicts of interests).
The NHSO and its chapters would have full-time paid staff providing logistical and content development for all matters before the NHSO. The budget of NHSO would be covered by user fees paid for by industry participants based upon company size.
Summary
The healthcare system in the United States is grossly inefficient. As long as consumers, practitioners, manufacturers, standards and guidance bodies, insurance carriers and government practices and policies are not coordinated, healthcare services will have excessive costs and will remain overpriced and the well-being of the overall public will remain inadequate.
The inefficiencies are largest in the area of chronic illnesses. Additionally, chronic illnesses represent the least likely areas for achieving an efficient outcome in a free marketplace given the complexity and the lack of readily-available information.
This paper shows why the public and its representatives in Congress should endorse a National Healthcare Standards Organization that builds on the seeds that are already blossoming in the fields of Parkinson’s disease and cancer. The National Health Standards Organization should focus on the Top 10 chronic illnesses to ensure all of the components of the healthcare industry are working in lock step and reflect the best interests of the public regarding these illnesses.


















