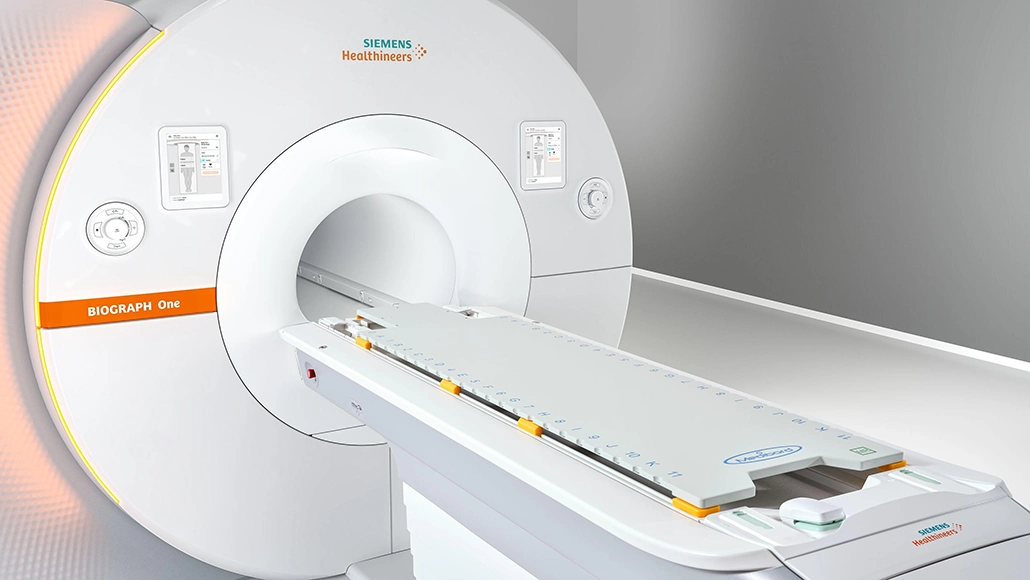
Siemens Healthineers FDA clearance for PET/MR scanner marks a significant step in advanced hybrid imaging, with the company receiving regulatory approval for its second-generation Biograph One PET/MR system.
The newly cleared PET/MR scanner enables simultaneous imaging of organ structure, function, and cellular metabolism. According to Siemens Healthineers FDA clearance for PET/MR scanner documentation, this dual-modality approach can support more precise tumour localisation, metabolic assessment, and treatment response monitoring, strengthening theranostics in personalised oncology care.
Siemens Healthineers said studies also suggest PET/MR could play a growing role in neurodegenerative disease assessment. Emerging drug therapies for conditions such as Alzheimer’s disease rely on PET imaging for diagnostic confirmation, while MR imaging is used to monitor therapy-related side effects.
Biograph One builds on the company’s first PET/MR platform, Biograph mMR, introduced in 2011. The second-generation system delivers faster image acquisition and more efficient workflows compared with earlier PET/MR scanners, while reducing patient scan times by up to 50 percent.
The system integrates PET and MR into a single workflow, addressing operational challenges associated with hybrid imaging. Siemens Healthineers said whole-body PET/MR exams, which traditionally exceed 60 minutes, can now be completed in under 30 minutes, enabling radiology departments to support twice as many patients.
The PET component is derived from the Biograph Vision PET/CT platform, using lutetium oxyorthosilicate detectors and a 35 cm axial field of view to reduce bed positions and accelerate whole-body imaging. MR technology is based on the Magnetom Vida 3 Tesla system, supporting high spatial resolution and flexible dose administration.
Several AI-driven features are embedded into the platform. The MyExam Companion interface automates scanning workflows for technologists, while Deep Resolve Boost image reconstruction accelerates acquisition, reduces noise, and improves image sharpness. BioMatrix Contour XL coils and an integrated BioMatrix Position Sensor further reduce setup time and technologist exposure.
“Over the past few years, we have seen a fundamental shift in personalized medicine. This shift has been driven by new drug developments, theranostics, and advances in technology, accompanied by a surge in the diagnosis of cancer and neurological diseases, especially Alzheimer’s disease,” said Katie Grant, head of Magnetic Resonance at Siemens Healthineers North America. “Patients experience a long, arduous pathway to answers and treatment. The Biograph One is the culmination of over a decade of experience in harnessing the diagnostic power of these two imaging worlds into one single modality, resulting in one truly patient-centric pathway for every single patient.”