GE HealthCare FDA clearance marks a key regulatory milestone for the company’s Allia Moveo interventional imaging system, which has received both U.S. Food and Drug Administration 510(k) clearance and CE Marking. The approvals support wider clinical adoption of the mobile platform across interventional and surgical environments.
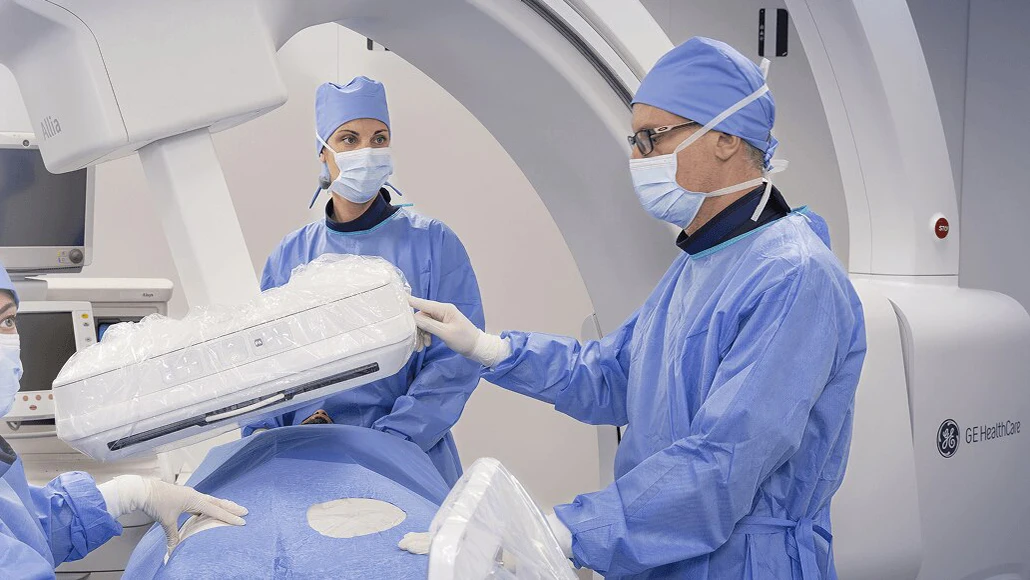
Allia Moveo is the latest member of GE HealthCare’s Allia line and was announced at the Radiological Society of North America’s 2025 annual meeting. Created for cardio, vascular, nonvascular, interventional, and surgical procedures, Allia Moveo merges a small form factor, cable-free C-arm with AI-assisted imaging and guidance technology.
GE HealthCare said that the system will free up space in interventional suites with its portability, quiet mobility system, and intuitive controls. These benefits aim to simplify workflows during minimally invasive procedures, which can involve numerous clinicians and imaging equipment.
The first global installation was completed at Hôpital Marie-Lannelongue, a national reference center for complex aortic disease. The hospital plans to use the system for vascular and cardiology procedures, including cone-beam computed tomography imaging. Its wide-bore design and flexible table movement are designed to accommodate patients of varying body size and weight while improving access for care teams.
“With Allia Moveo, we had this opportunity to position the system very quickly in any working position that will adapt the best to us,” said Stephan Haulon, head of the Aortic Center and Vascular Surgery at Hôpital Marie-Lannelongue. “One of the major milestones of this new system is having access to the reconstruction of the cone beam CT with CleaRecon DL, which is AI driven and gives you much better image quality.”
GE HealthCare stated that the system was developed through close collaboration with clinicians as procedures become more complex and interventional suite space becomes more limited.
“With Allia Moveo, we’re elevating the clinician experience, removing complexity, accelerating workflow, and enabling greater control during procedures,” said Arnaud Marie, general manager of interventional solutions at GE HealthCare. “We are excited to collaborate with leading experts at Hôpital Marie-Lannelongue on the first installation of this system, which is a defining step forward for care in the interventional suite, and a testament to our shared commitment to advancing meaningful innovation with the goal of supporting better outcomes for care teams and the patients they treat.”
Following the initial installation in France, Baylor St. Luke’s Medical Center became the first U.S. hospital to deploy the platform, extending GE HealthCare FDA clearance into clinical practice in the United States.
“We are honored to be the first hospital in the United States to install Allia Moveo, and proud to help advance the next generation of interventional care,” said Brad Lembke, president of Baylor St. Luke’s Medical Center. “This innovative platform enhances how our clinicians navigate complex minimally invasive procedures by improving mobility, image clarity, and workflow efficiency. It strengthens our ability to deliver precise, patient-centered care while supporting our teams with technology designed for the evolving demands of modern interventional medicine.”