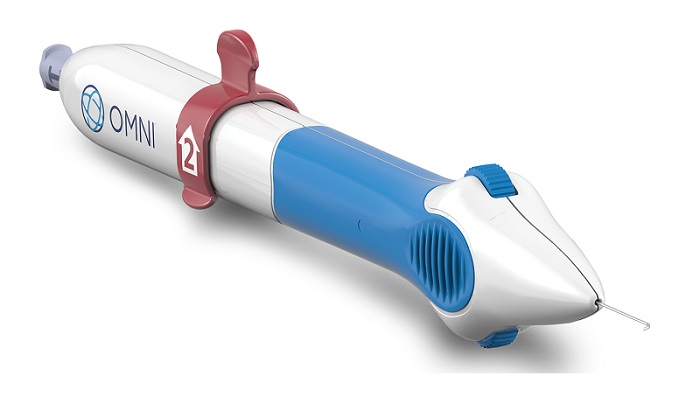
Sight Sciences, Inc., a growth-stage medical device company transforming the two fastest-growing segments in ophthalmology and optometry, glaucoma and dry eye disease, announced the launch of its next-generation OMNI® Surgical System for microinvasive glaucoma surgery (MIGS) procedures.
The OMNI Surgical System combines two distinct angle procedures – ab interno trabeculotomy and transluminal viscoelastic delivery. It is the only MIGS device that allows surgeons to target all three points of aqueous humor outflow resistance – trabecular meshwork, Schlemm’s canal and distal collector channels.
“We designed the next generation of our OMNI Surgical System to provide improved ergonomics, intuitive use and smooth microcatheter deployment and retraction, while continuing to deliver excellent outcomes as a standalone treatment, or as a procedure performed in conjunction with cataract surgery,” said Sight Sciences Chief Commercial Officer, Shawn O’Neil. “We’re excited to announce the availability of this improved system, which incorporates many changes based on feedback from our physician customers.”
The upgraded system features several updates designed to enhance its ergonomics and usability including:
An updated Luer lock fitting and pull pin to provide increased efficiency in the priming and preparation of the device,
An ergonomic handle and flexi-grip surface, which fits comfortably and provides stability in the surgeon’s hand, and
Enhanced gearing and intuitive wheel design that enables seamless deployment and retraction of the microcatheter.
“The OMNI Surgical System has been revolutionary for my practice because of its effectiveness and ability to offer titratable therapy to a wide range of patients with glaucoma,” said Blake K. Williamson MD, MPH, MS, refractive surgeon and MIGS expert at Williamson Eye in Baton Rouge, La. “These changes will improve the functionality and intuitive use of the OMNI System, making it even more comfortable and efficient to perform both viscodilations and trabeculotomies.”
Initial published results from early use of the OMNI Surgical System show meaningful reductions in intraocular pressure (IOP) and medication use.1 Several large-scale, multi-center retrospective and prospective MIGS clinical studies further evaluating the OMNI Surgical System are underway and in their advanced stages.
About OMNI® Surgical System
The OMNI Surgical System is a manually operated device for delivery of small amounts of viscoelastic fluid, for example Healon® or HealonGV® from Abbott Medical Optics (AMO), Amvisc® from Bausch & Lomb, or PROVISC® from Alcon, during ophthalmic surgery. It is also indicated to cut trabecular meshwork tissue during trabeculotomy procedures.
The OMNI System should not be used in cases where there is insufficient visualization of the anterior chamber. The following conditions may prohibit sufficient visualization required for safe and successful cannula and microcatheter placement: corneal edema, corneal haze, corneal opacity, or any other conditions that may inhibit surgeon view.
The OMNI Surgical System is a tool, not a treatment, and is indicated for use as specified above; it is not specifically cleared by the FDA to lower intraocular pressure in patients with open angle glaucoma.
About Sight Sciences
Founded in 2011, Sight Sciences has developed, and is now commercial with intelligently designed and engineered products that target the underlying causes of the world’s most prevalent eye diseases. The company’s surgical glaucoma product portfolio features the OMNI® Surgical System, a dually-indicated device that facilitates the performance of both trabeculotomy and transluminal viscoelastic delivery. Using proprietary multi-modal functionality, OMNI allows surgeons to target of all three sources of resistance in the conventional outflow pathway (trabecular meshwork, Schlemm’s canal, and collector channels) with a single device and single corneal incision.