An application that provides parents of sick and premature babies with video updates has just received central governance approval.
The seal of approval from NHS Digital means that the web-based application, vCreate, is now recognised by the NHS and will enable hospitals to roll out the service more quickly and easily across their Trust.
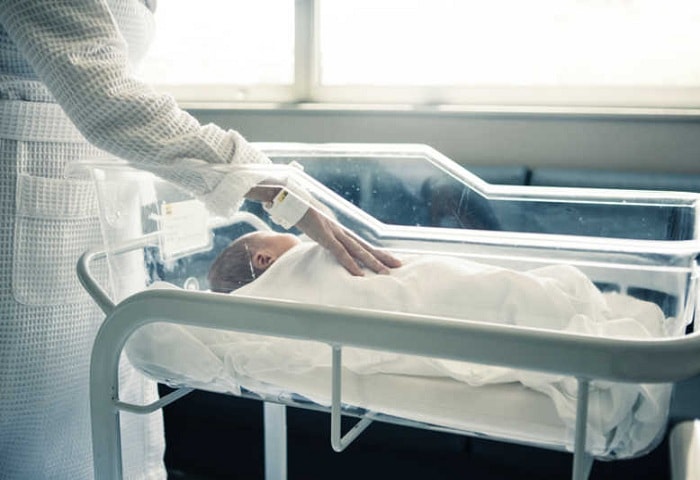
vCreate was first trialled at the Royal Hospital for Children, Glasgow, in April in the Neonatal Intensive Care Unit and now houses over 170 parents that have signed up to receive personalized video updates of their baby’s progress from their smart devices at times when they are not able to be at their baby’s cot side.
The technology allows nurses to record and send secure video messages to parents, to ensure that special moments that parents of premature infants would previously have missed, are captured on video.
When a baby is discharged, parents then have the option to download a secure baby care video diary that documents their baby’s journey and can be kept forever.
Ben Moore, Founder of vCreate, said: “We are thrilled to hear that vCreate has passed central governance and will now be listed as a trusted application. This long-awaited decision means that NHS Neonatal Units considering vCreate will now be able to get started more quickly, and parents with a child in an NHS neonatal unit will receive additional reassurance of their baby’s wellbeing when they need it most.”
vCreate is already in talks with over 100 neonatal units across the UK that are either using the platform or would like to. vCreate hopes that with NHS Digital’s central governance approval, more units will choose the application which is free to neonatal units and parents through an innovative funding model that sees private enterprises supporting the provision of the vCreate application in exchange for branding on the video page seen by parents.
In addition, to being the first app of its kind, vCreate has also pioneered a new approach to funding the application for neonatal units that it hopes to replicate in other areas of the NHS.
“Our aim is to make vCreate completely cost neutral to NHS Neonatal Units and parents using the service. We do this by working with individual hospital charities to find a local sponsor or benefactor that wants to fund the application for the hospital in exchange for branding on the video page seen by parents and positive PR,” commented Moore.
We’ve spent lots of time consulting with NHS consultants and now we think we have a model that benefits the NHS, as well as local private enterprises that want to become a force for good for their local neonatal unit.”