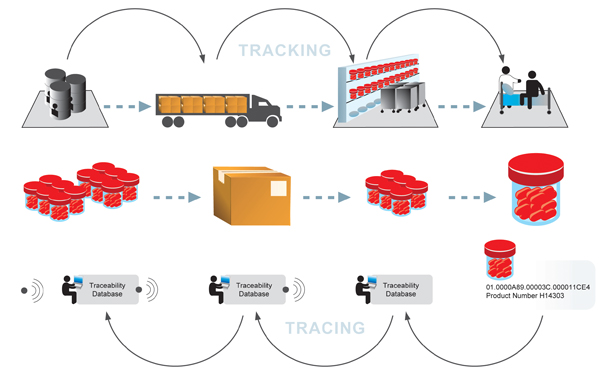
The 21st century has witnessed an active change in the working of the supply chain. As such, visibility is crucial in the healthcare industry where the emphasis on prompt delivery of treatments, under the right conditions is critical. Additionally, an assurance of quality is also of utmost importance in a dynamic regulatory environment
The “Darwinian Model” is alive and well. Significant risks and opportunities are driving businesses to shed the inefficiencies of the past or go the way of the dinosaur. This is especially true in Healthcare. Given the personal and professional impact of timely, cost efficient and most importantly effective treatments, today’s global healthcare industry faces enormous challenges and opportunities. Effectively trying to manage both ends of that spectrum is forcing executives out of the boardroom and into the supply chain. Here’s a look at what we’re facing.
Change is constant in the supply chain
Major evolutions in distribution and manufacturing in the 21st century have changed the dynamics of doing business in North America and across the globe. Growth in imports has soared in the last few years fuelled by the Asian domination of the manufacturing sector. This is especially true in North America where manufacturers have become importers and distributors with a new focus on efficiency and improved customer service.
Furthermore, with the shift in power due to margin compression, regulatory pressures and competing demand for capital in healthcare, many provider networks have taken their supply chains into their own hands while manufacturers have opted to focus on their core competencies; research and development, sales/marketing and customer services, delegating supply chain operations to outsourced experts. These movements in the landscape are at the heart of the changing economy and more so in healthcare where the management of a secure supply of healthcare products is vital for the well being of patients.The supply chain – a network of resources
The supply chain is no longer a controlled entity within the four walls of a warehouse. Today, it is a network of resources, scattered across facilities and entities in different cities and countries. To be effectively managed, supply chain resources need to be linked. Suppliers, partners and customers; each performing a role in the supply chain, and each user and/or automated process are small “hubs” contributing to the movement of goods, funds as well as information in the supply chain.
The need for visibility
To support today’s business model in this high-velocity, complex and distributed logistics environment, real-time visibility has become a key strategic imperative. Visibility to suppliers’ production rates and shipment lead times, in-house inventory, historical data, and customer sales projections can drive benefits in efficiency, lower inventories and improve fulfillment rates. Overall, visibility is driven by companies’ need to:
- Become more proactive and systematic in their supply chain operations
- Track and trace products throughout the supply chain, from cradle to grave
- Proactively alert customers of product availability and status of shipments
- Improve on-time delivery, reduce lead time and lead time variability
- Reduce and/or redirect working capital, as well as fixed and variable costs
These are fundamental capabilities for the supply chain today, and given the risk and regulatory oversight, clear visibility is vital in Healthcare!

With over $4.5 trillion in expenditure, the global medical industry is one of the world’s largest and fastest growing industries, comprising various sectors: medical equipment and supplies, pharmaceutical, healthcare services, biotechnology, and alternative medicine sectors. Undoubtedly, the management and delivery of these vital goods throughout the healthcare supply chain are proportionally as complex and important as its size and velocity.
Overall, the process of manufacturing and distributing pharmaceutical products is similar to that of other industries. Companies purchase raw materials for bulk synthesis of active and inactive ingredients. Dosages are formulated and packaged. Products flow (from cradle to grave) through manufacturers’ warehouses, wholesale distributors/3rd Part logistics providers, retail pharmacies, medical institutions, and finally to the patient. Some products make their way back to their manufacturers due to recalls and returns.
Today, the healthcare industry is characterized by a number of drivers affecting its supply chain, including:

- Globalization, competition and margin compression
-
Increased regulatory oversight
-
The rise in IT budgets at healthcare institutions
-
Growth in usage of medication
-
Increased cost of drug development, production and distribution
-
Major retailers driving packaging and labelling requirements
-
Manufacturers’ desire to control the customer and margin away from wholesalers
-
New outsourcing models in the “patent to patient” supply chain process
To meet these while improving cost, reducing inventory and maintaining high fill rates is a significant challenge to any supply chain. It is an even greater challenge in pharmaceuticals because of the compliance and regulatory requirements.

Visibility in healthcare:
Visibility in healthcare is certainly vital! Not knowing the route by which pharmaceutical products make their way to the consumer can lead to risk in counterfeits. In addition, with FDA regulations, life science companies need to trace drug and product information such as historical locations; time spent at each location, record of ownership, transaction history, packaging configurations, and environmental storage conditions to efficiently and safely manage the full lifecycle of such products in the supply chain. Major drivers for visibility in healthcare include the need to:
- Meet Pedigree requirements and deal with counterfeits
- Manage Product recalls
- Deal with offshore manufacturing quality
- Identify and mitigate diversion
Pedigree
A drug pedigree is a statement of origin that identifies each prior sale, purchase, or trade of a drug, including the date of those transactions and the names and addresses of all parties to them. Under the pedigree requirement, each person who is engaged in the wholesale distribution of a prescription drug in interstate commerce, who is not the manufacturer or an authorized distributor of record for that drug, must provide to the person who receives the drug a pedigree for that drug.
Recalls
According to the FDA, there were over 140 drug recalls in 2007. Similar to the food industry, when a recall is made, drug companies suffer twice: once from the massive logistics effort to pull their product from the supply chain and the second from the substantial negative impact on consumer confidence. Faced with a significant number of product recalls, the healthcare industry is seeking ways to better monitor the international drug supply from “raw material to consumers.”
Counterfeiting in the supply chain
The pharmaceutical industry also suffers from drug counterfeiting, in 2007 there were thirty investigations by the FDA. Visibility to origin and ownership of products could help prevent counterfeit drugs from reaching consumer markets.
Offshore Drug Manufacturing
With the rise of the number of generic products and their lower margins, manufacturers are driven to go offshore to improve their competitiveness and profitability. This has created thousands of offshore healthcare product manufacturers in environments where strict adherence to six sigma processes is extremely difficult to manage.
Diversion
Diversion is an unauthorized channel in the supply chain and is another form of counterfeiting. Channel corruption within the supply chain can distort profits and revenue distribution which often leads to additional black market activities and diminished brand value, particularly in Europe where borders are becoming virtual.
To ensure the validity of data in the pharmaceutical industry, healthcare companies are looking to traceability and visibility for reducing costs, while improving product quality and drug safety.
As CEO, I need to feel the pulse of my organization; where the challenges are, what is working for us, where we need to do further work, where we need to invest, where to lower cost. In brief, I need key performance indicators that represent realistic goals to my management and enable them to manage with confidence based on an up-to-date view of our operations.
With the right visibility tools such as TECSYS’ iTopia®, manufacturers, distributors and 3PLs are empowered to manage supply chain issues related to track and trace requirements for their healthcare products, and as importantly manage their supply chain operations more effectively.
What is iTopia? No…not another new drug! But it maybe a good medicine to remedy visibility issues in your healthcare supply chain.

Manufacturers believe that track-and-trace technologies will enable increased operational efficiency through improved product visibility, chargeback and returns processing, product recall ability and product security.
Similarly, distributors and retailers see track-and-trace implementation primarily as a tool to achieve operational efficiencies. They believe the use of track-and-trace technologies will play a key role in future practices and processes on a wide scale, but will need to be phased-in for supply chain-wide adoption.
Through automatic identification, traceability and visibility tools, healthcare companies are given the opportunity to make the Healthcare supply chain more efficient and accurate, and thus safer by:
- Reducing medication errors
- Making counterfeiting more difficult
- Enabling efficient and effective traceability
- Decreasing the production and supply chain cost
Auto-ID and visibility technology can help manage risk and maintain pedigree by tagging pharmaceuticals and product packaging with barcode or radio frequency identification (RFID) tags. This allows products to be tracked, traced and recalled if necessary.
However, up till now, the lack of standards and imperfect capture rate of RFID readers are a major concern to manufacturers. To move forward, serialization at the unit level will be required, but how that shakes out in the coming months and years, remains to be seen. At this point in time, bar-coding seems to make the most sense; some manufacturers have already taken that route for tracking and tracing products in their supply chain.
In addition to track-and-trace, the most common visibility applications include:
- Order Inquiry with drill-down by customer, item, PO, order date and the like
- Inventory inquiry to aggregate inventory from multiple warehouses into a single view
- Purchase orders visibility to view and update purchase order status from issue to delivery
- Dock scheduling to maintain inbound and outbound dock schedules for multiple warehouses

Visibility systems must also include powerful event-management capabilities that can notify the appropriate individuals important key events occur which impact their decision processes; such as a product alert, a customer emergency, an inventory shortage or a delay in delivery. Users must have the flexibility to set event triggers and communication methods with for e.g. RIM, sms, e-mail, and other electronic forms of communications.Powerful notification functionality helps keep operations on track. When an exception is detected, an alert is sent out to the relevant decision makers who can then use available tools to address the ramifications of corrective action. Distribution, transportation, and logistics are also integrated into this real-time process to support smooth and efficient supply chain.
From an individual user’s perspective:
Visibility tools are about providing intelligence through the transparent access to information anytime, anywhere, and by making this information easy to use by filtering out what is not important and by focusing on key indicators relevant to the specific user. These tools, such as TECSYS’ iTopia, have the ability to extract information from disparate, sometimes hard to get at, back-end enterprise systems, pulling relevant information down to the desktop or mobile computing device where it can be consolidated, manipulated, graphed and shared with others. With intelligent visibility tools:
• Executives are able to determine where the risks are and where the opportunities lie.
• Customers may be able to access views to track their own orders, as well as check on inventory levels and reconcile outstanding credits or invoices.
• Vendors are able to see how their inventory is moving within the warehouse.
• Sales representatives are equipped with real-time information and always able to answer questions on product, stock and pricing.
• Customer service reps are able to deal with critical shipments, from tracking to status of orders.
Deploying visibility in healthcare
For years, healthcare organizations have had fragmented IT environments with multiple applications and silos of data that cannot be quickly and easily accessed—resulting in operational inefficiencies, increased costs decreased service levels and risks to patients. Certainly, visibility tools should be at the forefront of healthcare technology adoption.
However, the high cost, time required and internal political struggles are often the biggest barriers to integrate disparate systems of healthcare IT. Healthcare organizations have primarily two choices:
- Adopt Enterprise Application Integration (EAI) suite to tie together and capture all relevant data in disparate applications.
- Outsource to 3PLs: 3PLs provide an asset and services platform for the management and visibility of healthcare supply chains. It is a distribution and IT infrastructure typically built and maintained with a focus on standards, regulatory compliance and best-practice distribution, business intelligence and track and trace technology. With 3PLs, healthcare products manufacturers are able to achieve:
- Scaling:
Given manufacturers’ expanding product portfolio, internal supply chain resources are not able to effectively scale. With 3PLs shared utility model, manufacturers are able to leverage the already established and maintained supply chain technology infrastructure and visibility tool to enable their business operation’s expansion. - Risk Mitigation:
Companies that have traditionally distributed out of a single location face increasing pressure to develop contingency capabilities. Given the dollar value of products in their supply chain, especially in controlled substances and cold chain products, the need to mitigate risk drives the need for outsourcing. - Focus:
While dealing with several of the pedigree issues related to their products, manufacturers the opportunity to focus resources, reduce costs and improve quality by leveraging 3PLs established infrastructure. - Benchmarking:
As a shared utility, typically 3PLs are able to sustain the healthcare’s industry best practices with greater frequency and ability than a manufacturer. This is especially true from a Quality and Regulatory affairs and IT perspectives.
Benefits of Investment in visibility tools and technology
With visibility tools, and after addressing pedigree requirements, Life science companies can reduce volume of returned product and reduced costs for product returns and recalls. They can also address inventory management efficiencies, improve forecasting capabilities, reduced out-of-stock items, increase service levels, and achieve reduction in overall supply chain operating costs. Below are examples of financial benefits sited at some companies who have deployed visibility tools.

Examples of benefits sited include:
- Improved visibility such as real-time visibility into customers orders
- Improved responsiveness to customer inquiries and supply chain exceptions
- Improved efficiency such as minimizing inventory and improved margins
Moving forward
Undoubtedly, challenges and changes will be constant in the patent-to-patient supply chains. Evolutions together with the full-effect of globalization have increased the “check points” and therefore vulnerability in the supply chain process; adding risks and uncertainties to suppliers, consumers and regulatory bodies.
While it is important to acknowledge the imperfect world surrounding the healthcare supply chain, we; whether manufacturers, distributors, 3PLs or retailers, are today empowered with advanced technology, best practice distribution processes and regulatory standards to make major strides forward in mitigating much of the challenges we are facing in our daily management of the supply and demand activities.
Management’s ability to achieve a nearly risk-free environment is primarily enabled by visibility technology that provides intelligence into every step of the healthcare supply chain. It is facilitating decision making and heightening the awareness level to effectively manage exceptions!
As a CEO in healthcare supply chain management, avoiding extinction requires that I get out of the boardroom and into the supply chains of my customers. Having the right data and visibility tools allows us seize opportunities and mitigate risks. It’s all about control. Having the control and extending that control are key to the value that we bring to ourselves and ultimately to our clients and patients.
/* Style Definitions */ table.MsoNormalTable {mso-style-name:”Table Normal”; mso-tstyle-rowband-size:0; mso-tstyle-colband-size:0; mso-style-noshow:yes; mso-style-priority:99; mso-style-qformat:yes; mso-style-parent:””; mso-padding-alt:0in 5.4pt 0in 5.4pt; mso-para-margin:0in; mso-para-margin-bottom:.0001pt; mso-pagination:widow-orphan; font-size:11.0pt; font-family:”Calibri”,”sans-serif”; mso-ascii-font-family:Calibri; mso-ascii-theme-font:minor-latin; mso-fareast-font-family:”Times New Roman”; mso-fareast-theme-font:minor-fareast; mso-hansi-font-family:Calibri; mso-hansi-theme-font:minor-latin; mso-bidi-font-family:”Times New Roman”; mso-bidi-theme-font:minor-bidi;}